Bionic creation of nano-engineered Janus fabric for selective oil/organic solvent absorption
Abstract
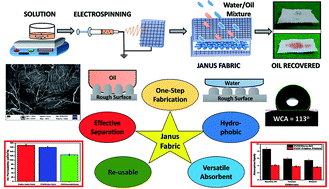
In this study, we present a self-driven and tunable hydrophobic/oleophilic, wettability-modified Janus fabric composed of a cellulosic substrate engineered with nanofibers via facile electrospinning technique that exhibits one-step selective oil absorption capacity from water. A nano-fibrous porous non-woven mat of polyvinylidene fluoride (PVDF) is coated on the cellulosic substrate with and without inclusion of silicon carbide (SiC) nanoparticles. PVDF and nano-SiC particles facilitate the hydrophobicity (WCA 113° ± 1.6°), and at the same time the porous structure and high aspect ratio of the nano-fibrous mat support the superoleophilicity (WCA 0°). Morphological analysis and air permeability studies revealed the corroboration of porous fine interconnected nanofibers. The retrieved Janus fabric efficaciously separated the oil/solvent from water with a constant selective oil absorption capacity up to 8.6 times, 5.9 times, and 5.5 times against its own weight for machine oil, toluene and ethanol respectively. It is also observed that the SiC nanoparticles augmented the absorption capacity in the Janus structure. Furthermore, the engineered Janus fabric can be reused up to 10 times during the oil/solvent recovery. The reported Janus fabric possesses the advantage of scalable fabrication, high separation efficiency, stable recyclability, excellent durability, time saving, and has strong potential for industrial applications in oil spill management.

 Please wait while we load your content...
Please wait while we load your content...