DNA assembled metal nanoclusters: synthesis to novel applications
Alok Pandya
*a,
Amitkumar N. Lad
*b,
Surinder Pal Singh
c and
Rishi Shanker
a
aDivision of Biological & Life Sciences, School of Arts & Sciences, Ahmedabad University, Ahmedabad, 380 009, Gujarat, India. E-mail: alok.pandya@ahduni.edu.in
bGujarat Forensic Sciences University, Institute of Research and Development, Sector 18A, Gandhinagar, 382007, Gujarat, India
cNational Physical Laboratory, Dr. K. S. Krishnan Marg, New Delhi – 110012, India
First published on 21st November 2016
Abstract
DNA based nanostructures provide a versatile foundation because they exhibit fascinating luminescent and detection properties. Among such DNA nanostructures, DNA assembled fluorescent metal nanoclusters (NCs), such as silver (Ag), gold (Au) and copper (Cu) nanoclusters, as a new class of fluorophore have attracted progressively more attention owing to their unique electronic structures and the subsequent unusual physical and chemical properties. These excellent properties make them ideal luminescent probes for bio-chemical applications. Blending of metal with DNA sequences as a stabilizing agent has generated a large number of biosensors, as well as imaging and catalytic properties. To date, significant efforts have been devoted to the synthesis, properties and application studies of DNA hosted Au, Ag and Cu NCs. In this review article, we focus on summarizing recent advances in controllable synthesis of DNA, nucleotide-templated NCs strategies and emerging applications of metal NCs, including Au, Ag and Cu. Finally, we conclude with the enduring goal of this area of research, which could explore as novel promisingly environmental-benign approaches to construct nano clusters through DNA-based conjugation and modulation.
1. Introduction
Recently, a new class of nanomaterials that bridges the gap between traditional organometallic compounds and crystalline metal nanoparticles, called nanoclusters (NCs), has emerged owing to their excellent characteristics, such as biocompatibility, photo-stability, sub-nanometer size, optical properties and easy synthesis.1 Metal nanoclusters (NCs) have gained special attention owing to their attractive features like the absent link between metal atoms and nanoparticles (NPs), which exhibit distinct optical and plasmonic properties,2–7 and also display molecule-like behaviour.8–12 In bulk metal, the conduction band has no energy gap separating it from the valence band, so electrons move freely. The scattering of the electrons is determined by the electrons' mean free path. In contrast, in metal NPs, the size is comparable to or smaller than the electron mean free path, where the motion of electrons becomes limited by the size of the NP and interactions are expected to be mostly at the surface.13–16 This gives rise to surface plasmon resonance effects. However, in metal NCs, the size of the metal is usually 1 nm (few to a hundred atoms) and the continuous band structure becomes discontinuous and is broken up into discrete energy levels. The pioneering work of the Dickson group paved the way for routine preparation of NCs in aqueous solution with a number of polymeric or thiol templates, including dendrimers,17–22 proteins,23 DNA,24,25 and GSH.26–28 This bioconjugation provides extra functionality to nanoclusters, such as stability, biocompatibility, and targeting. Generally, fluorescent gold nanoclusters are protected by the carboxylic acids, which can conjugate with amino-molecules to form stable amide bonds catalyzed with a carbodiimide or sulfo N-hydroxysuccinimide ester. Besides the covalent conjugation, proteins can also be directly adsorbed onto negatively charged nanoclusters through electrostatic interaction,27 as illustrated in Fig. 1. | ||
| Fig. 1 Schematic illustration of bioconjugation of metal NCs. (a) Use of a bifunctional ligand such as a mercaptoundecanoic acid derivative for linking fluorescent Au nanoclusters to biomolecules. (b) Positively charged biomolecules are linked to negatively charged nanoclusters by electrostatic attraction. (c) Covalent linkage of amide bond via EDC chemistry. (d) Oligocytosine encapsulation of fluorescent Ag nanoclusters. (This figure has been reproduced from ref. 27 with permission from Springer.) | ||
2. Metal nanoclusters and their detection strategy
It is well known that nanoclusters are formed by the reduction of metal ions in the presence of suitable reducing agents. Nevertheless, under these conditions, metal NCs are strongly prone to interact with each other and aggregate irrevocably to reduce their surface energy. As a result, an appropriate stabilizing scaffold is essential for the production of metal NCs.28 Moreover, the nature of the scaffold is also responsible for their sizes and fluorescence properties.29,30 The fluorescent nanoclusters (NCs) of silver (Ag), gold (Au), magnesium (Mg) and copper (Cu) have emerged as novel materials for biosensor and bioimaging development31,32 (Fig. 2). In contrast to semiconductor quantum dots, metal NCs conjugate more easily to biopolymer molecules with minimal concern for toxicity.33,34 Such bio-conjugations are usually based on passive adsorption, multivalent chelation and covalent-bond formation. Therefore, in order to understand bioconjugation with affinity ligands, functionalization of fluorescent metal NCs is necessary with reactive functional groups, such as primary amines, carboxylic acids, alcohols, or thiols. Example such as, 11-mercaptoundecanoic acid (MUDA)–ligand exchange, and polyamidoamine (PAMAM) templated AuNCs on functionalization with thiol, enabled their conjugation with a specific peptide named the SV40 nuclear-localization signal (NLS).35 In another report, poly(acrylic acid) (PA)-stabilized AgNCs were transferred to anti-actin Ab/C12 and anti-a-tubulin/C12 conjugates for cell-surface labelling. Besides the covalent conjugation and ligand exchange, direct adsorption of proteins onto negatively charged NCs through electrostatic interactions was also used in metal-NC bioconjugation. A breast-cancer marker protein, platelet derived growth factor, was readily conjugated with AuNCs via electrostatic and hydrophobic interaction.36 Owing to the aforementioned effort in the development of new fluorescent probes, chemists and biologists are now focusing on developing new fluorescent tools in terms of size, toxicity, cost, emission line-width, brightness, chemical and photostability, bioconjugate friendliness, environmental sensitivity, fluorescent active, and multi-color capability. Among those water-soluble gold or silver nanoclusters developed in the past decade, DNA-templated metal nanoclusters have received increasing attention due to a number of useful properties, which are described in the next section.2.1 Fluorescent metal nanoclusters
Oligonucleotide templated nanoclusters have attracted increasing attention owing to their facile synthesis, tunable fluorescence emission, high photostability, and suitability as a diagnostic tool for biological concerns.37 Coupling of these DNA stabilizers with other sequences, such as aptamers, has generated a large number of biosensors. Coupling of the excellent photoluminescence (PL) features with the functionality of the host DNA matrix provides immense potential for chemical and biological sensing. This review focuses on DNA-templated NCs as DNA is an interesting ligand for preparation of fluorescent metal NCs with applications in biosensing.2.2 Electrochemical metal nanoclusters
Electrochemical labels are commonly used to amplify the signal output and a variety of nanomaterials are increasingly employed as effective signal enhancers because of their unique electronic and catalytic properties, as well as their high specific surface area and the high loading capacity for the receptor molecule.76,87 The very few attempts that have been made at exploring the NCs based options in electrochemical detection point towards the undiscovered applications in medicine and the environment.2.3 Why DNA?
DNA is perhaps one of the most interesting ligands for preparation of fluorescent metal NCs for sensing applications. Some significant attributes are:(1) It shows different emission colors ranging from blue to near infrared (NIR) with high quantum yield (QY), simply by changing the DNA sequence. In fact, DNA encodes the structure and the function of AgNCs, which is more difficult to achieve with other types of polymer.
(2) DNA has an excellent molecular recognition function and it binds to complementary nucleic acids. DNA aptamers can selectively target diverse range of analytes, e.g., metal ions, small molecules, proteins and cell-surface receptors.38–43 New biosensing platforms can be achieved by reasonably designing DNA sequences to combine molecular recognition and NC-templating properties.
(3) Arbitrary length and sequence of DNA can be modified by chemical synthesis for selective targeting. It is more difficult to achieve flexibility of control compared to other types of polymer. Furthermore, it is also advantageous to use DNA for systematic mechanistic studies and sensor optimization with their high stability.
(4) Unlike organic fluorophores or semiconductor quantum dots, the NCs do not need covalent modifications on DNA, making synthesis more cost effective. NCs also have fewer concerns regarding toxicity than QDs.
The niche of metal NCs has grown rapidly and addressed physical chemistry, synthesis, DNA sensing, and other applications.44–55 This review discusses the recent progress in the use of DNA-templated metal NCs for the detection of metal ions, small molecules, proteins, cell-surface receptors and cell imaging. In particular, we focus on the mechanistic understanding, sensing and other emerging applications that will significantly impact future research on this new classes of nanomaterials.
3. DNA-templated metal nanoclusters and synthesis
DNA nanotechnology has emerged as an excellent vehicle for the controlled assembly of nanoparticles because it enables the positioning of particles with nanoscale precision and the tailoring of their binding interactions.56 While there are simpler implementations, where DNA particle assembly involve controlled nanoparticles aggregation with construction of well-defined clusters and lattices.57,58 For example, micrometer-scale dielectric particles have been assembled into tetrahedral and octahedral clusters. In other schemes, trimer clusters, tetrahedral clusters, chiral helical assemblies, and two and three dimensional lattices of plasmonic nanoparticles have been constructed.59–62 The properties of such DNA assemblies have drawn attention towards the facile synthesis of oligonucleotide templated nanoclusters (Fig. 3).In such syntheses, ligands are critical for the synthesis, stabilization and control of the electronic properties of metal nanoclusters.63–67 Over the past decade, DNA has been increasingly used as a ligand to prepare silver,24,25,68–72 copper73 and platinum74–76 nanoclusters with interesting luminescent, detection, and catalytic properties.77 DNA is a natural nanoscale material with a strong affinity for metal cations.78 DNA can also template and localize metals to form and stabilize NCs. In addition, exquisite control of NC size and the resulting electronic and optical properties have made DNA the natural ligand choice for NC synthesis and their various applications (Fig. 4). Finally, the chemistry of DNA-templated NCs can be performed in water and neutral conditions, which is a green and desirable method for technology development as opposed to organic solvents or acidic/alkaline reaction conditions. Further, the synthesis of different DNA-templated Au, Ag and Cu nanoclusters will be addressed.
3.1 DNA-templated Au nanoclusters
DNA oligonucleotides have been widely used as the scaffold for preparing metal NCs with Ag, Au and Cu. In comparison to the successful strategies for the DNA-templated synthesis and optical tunability of silver nanoclusters (AgNCs), there are very few reports on the synthesis of gold nanoclusters (AuNCs) with DNA as the template79–84 (Fig. 5). Chen et al. reported biomolecule-supported atomically monodispersed AuNCs directed by DNA as the etchant for gold nanoparticles and rods.81 Firstly, Liu et al. showed that pH-dependent single-stranded DNA (ssDNA) was feasible in synthesizing blue fluorescent AuNCs with a mild reductant.85 Recently, water-soluble and red-emitting AuNCs were synthesized with single-stranded DNA as a favourable bio-template and dimethylamine borane as a mild reductant.86 Single-stranded DNA was first reported as a substitute template during reduction of an Au precursor to produce AuNCs. Most recently, the synthesis and characterization of a new DNA templated gold nanocluster (AuNC) integrated with bilirubin oxidase (BOD) and single walled carbon nanotubes (SWNTs) was reported with ∼1 nm diameter and possessing ∼7 Au atoms.87 These AuNC act as an enhancer of electron transfer (ET) and lower the over potential of the electro-catalytic oxygen reduction reaction (ORR) by 15 mV as compared to the enzyme alone, which causes significant enhancements in the electro-catalytic current densities at the electrode. It was shown that such enrichment of ORR by the AuNC is only explicit to nanoclusters and not to plasmonic gold nanoparticles. In another report, DNA-templated blue-emitting fluorescent gold AuNCs were prepared and further reduced to red emitters using Good's buffers, which were found to be useful reducing agents for promoting this conversion.88 Dimethylamine borane (DMAB) is responsible for red emission. Poly-C DNA produces AuNCs only at low pH and each DNA chain can only bind to a few gold atoms, regardless of the DNA length. Otherwise, it may result in large nonfluorescent gold nanoparticles (AuNPs). Each poly-A DNA might template a few independent AuNCs. West et al. reported the synthesis of DNase 1 stabilized gold nanoclusters (DNase 1:Au8 NCs) with core sizes consisting of either 8 or 25 atoms, which exhibit blue fluorescence, whereas the DNase 1:Au25NCs are red emitting. Furthermore, the synthesized DNase 1:AuNC has been used for simultaneous detection and digestion of DNA with a detection limit of 2 μg mL−1.89 Recently, Li et al. prepared poly-adenine-templated AuNCs with excellent fluorescent properties, which demonstrates that pre-incubation of poly-adenine with HAuCl4 is unnecessary in the preparation of poly-adenine-templated gold nanoclusters (AuNCs).90 | ||
| Fig. 5 Schematic representation of colorimetric detection of ToLCNDV DNA using AuNP conjugated bi-functional oligonucleotide probe and self-assembly of gold clusters. (This figure has been reproduced from ref. 79 with permission from the Royal Society of Chemistry.) | ||
3.2 DNA-templated Ag nanoclusters
The strong interaction between DNA and Ag+ was evident in gel electrophoresis.91 Binding of Ag+ to DNA induces a conformational change in DNA and causes a mobility decrease during the electrophoretic processes. Silver ions show strong affinity to DNA scaffolds by binding to their heterocyclic bases rather than to the phosphate or sugar groups. The existence of one C–Ag+–C base pair in duplex DNA was reported to increase the melting temperature to 81 °C and was consistent with the high stability of the C–Ag+–C base pair.92 In addition, density functional theory (DFT) calculations showed that binding of Ag+ to the N3 of C has the lowest binding energy over other DNA bases,93 again supporting the strong interaction between Ag+ and C base. The strong binding affinity between Ag+ and C in C-rich DNAs with inside C–C mismatching pairs has been exploited in the development of molecular Ag+ sensors94–96 and to design some special DNA structures.97 Therefore, the strong binding affinity to Ag+, as well as the flexibility and variety make it possible to create AgNCs with DNA scaffolds. Typically, a solution mixture of DNA and Ag+ is first incubated for an appropriate time at low temperature to form DNA–Ag+ complexes, followed by the reduction of NaBH4.24,25 The synthesis of AgNCs with DNA scaffolds is relatively simple without the formation of large nanoparticles.The first report on the synthesis of DNA–AgNCs from Ag+ using a 12-base scaffold of 50-AGGTCGCCGCCC-30 was published in 2004.24 It was found that Ag+ favours association with the heterocyclic bases over the phosphate groups. The proportion of Ag per DNA strand varies from one to four, which leads to different electronic transitions being observed in the fluorescence spectra. In fact, NMR spectra showed that C bases have the largest chemical shifts upon interacting with AgNCs. A bright DNA–AgNCs template in phosphate buffer solution with near infrared (NIR) emission was synthesized.98 The oligonucleotide C12 templated DNA–AgNCs were synthesized and exhibited a range of fluorescence and absorption spectra.99 Mass spectra also showed various forms of oligonucleotide species with 2–7 Ag atoms. Using DNA templates with a combination of sequences, five types of fluorescent DNA–AgNCs were synthesized with maximum emission wavelengths ranging from 485 to 705 nm.100 The effect of the DNA sequence and length on the fluorescent properties of the DNA–AgNCs were investigated through synthesis of five AgNCs using NaBH4-mediated reduction of Ag+ in the presence of various DNA scaffolds. Mass spectra of DNA–AgNCs confirmed that 2–6 Ag atoms were present in these DNA scaffolds and revealed that the sequence and length of the DNA scaffolds might play an important role in determining the size of the AgNCs.
It is usually accepted that the conformation of DNA scaffolds is related to the affinity between the Ag+ and the DNA bases. Consequently, the secondary structure of DNA scaffolds has an important effect on the structure and optical properties of DNA–AgNCs. Several different secondary structures (such as hairpin, i-motif and G-quadruplex) of ssDNAs have been used to synthesize DNA–AgNCs. Recently, it was found that DNA–AgNCs can be prepared using C-loop containing hairpin DNA scaffolds,101 and both the C-loop and the G-loop showed bright fluorescence. Those having the A-loop and the T-loop exhibited weak or no emission under excitation at visible wavelengths. A C-loop structure was introduced into a self-assembled DNA nanotube to yield fluorescent AgNCs and, interestingly, it showed nearly identical fluorescence spectra to the resultant AgNCs on hairpins. Moreover, the loop sequence, length and stem sequence also influence the synthesis of AgNCs (Fig. 6). A hairpin DNA structure with high brightness was demonstrated102 by stem-directed growth of AgNCs. The i-motif is an unusual form of C-rich DNA, which is formed through the intercalated C–C+ base pairs. It is one of the known nucleic acid structures involving systematic base intercalation.103 Further, AgNCs were successfully synthesized with i-motif DNA.104 Two i-motif-forming oligonucleotide-stabilized ((TA2C4)4 and (C4A2)3C4) AgNCs showed red and green emissions, which are favoured in slightly acidic and basic solutions, respectively. By using inter/intramolecular i-motif DNA, fluorescent DNA–AgNCs were successfully prepared with an emission wavelength range over green to NIR.105 G-Quadruplexes are another example of specific ssDNA structures, which is a higher ordered DNAconformation based on the Hoogsteen hydrogen bonding among four guanine bases with a nearly square planar structure.106 The dual-emissive DNA–AgNCs were synthesized using a specific G-quadruplex DNA sequence (AS1411), which demonstrated that AS1411 retains its G-quadruplex structure in the complex of AgNCs.107 Owing to the great stability of the intermolecular G-quadruplex structure, red-emissive DNA–AgNCs show high thermo-stability.
 | ||
| Fig. 6 Schematic of DNA nanotube with hairpin protrusions. Top left: Tube composed of tiles, with hairpins represented by red asterisks. Top right: Axial view showing outward orientation of the hairpins. Bottom: Tile, arrows point to the 3′ end. The hairpin region of strand no. 1 was omitted to create bare tubes. (This figure has been reproduced from ref. 101 with permission from the American Chemical Society.) | ||
The ssDNA has a more rigid structure and a well-defined conformation owing to duplex DNA, which offers the possibility of designing specific binding sites of AgNCs. However, the fully complementary dsDNA does not provide sufficient space to accommodate the binding of AgNCs. Such defects in dsDNA are always necessary to form dsDNA–AgNCs. Particularly, DNA scaffolds with C–C mismatches are interesting owing to stable C–Ag+–C coordination. Owing to such properties, AgNCs were successfully synthesized using an inner C6-loop-containing dsDNA scaffold through the formation of a duplex DNA structure with DNA containing the sickle-cell anemia gene sequence.108 The upstanding C6-loop provided a binding site for the generation of yellow-emissive AgNCs. Base mismatch is one of the common defects among DNA damage, which can cause diseases. Base mismatch on dsDNA scaffolds can endow them with a binding site for Ag+, which facilitates the formation of AgNCs using dsDNA scaffolds. With the introduction of a single mismatched base pair at the G–C position, fluorescent AgNCs were synthesized with dsDNA.109 AgNCs-functionalized DNA nanowires or hydrogels with better thermo-stability were constructed using specific terminal sequences.110,111 The presence of abasic sites (AP sites) is correlated with cellular viability and genomic integrity because AP sites lead to mutations in DNA replication. Fluorescent AgNCs were successfully synthesized using dsDNA containing an AP site as the scaffold.112 The fluorescence intensity of AgNCs decreases upon contact with mismatched DNA. Apparently, the fluorescence decreases when the DNA strand contains T or A at the mismatch position, in comparison to a smaller decrease for a G or C mismatch. Another common DNA defect is the abasic (AP, apurinic/apyrimidinic) site, which occurs spontaneously at an extensive rate by DNA glycosylases during the removal of damaged or incorrect bases from DNA.113 This study also suggested that the size of produced AgNCs is not affected by increasing the Ag+ concentration, and the emission is robustly dependent on the base stacking direction of DNA scaffolds.
DNA origami nanostructure conformation has shown specific two- and three-dimensional shapes during nanoscale folding of DNA.114 The advancement of DNA origami nanotechnology allows the design and construction of nanometer sized DNA structures with accurate cavities or gaps. These special DNA nanostructures pledge the synthesis of nanomaterials with narrow size distribution and controllable site specificity. The site-specific synthesis of AgNCs with careful design and in situ immobilization was achieved on a triangular DNA origami scaffold by the Tollens reaction.115 By connecting the sugar motif into DNA, this approach does not require the traditionally used reductant (NaBH4), which can reduce disulfide bonds and potentially affect the function of proteins. The as-prepared AgNCs have bright blue emission, excellent photostability and narrow size distribution. Furthermore, DNA–AgNCs can also be prepared via direct synthesis through template conversion.116 The weakly fluorescent AgNCs were first prepared using polyacrylic acid (PA) as a template. Upon addition of ssDNA, the prepared AgNCs rapidly transferred from PA to DNA binding, leading to enhanced fluorescence intensity. As a result of the formation of DNA–AgNCs, solution pH, buffer conditions and temperature affected the transfer efficiency.
3.3 DNA-templated Cu nanocluster
Newly emerging Cu nanoclusters, which selectively form on DNA duplexes, offer excellent potential as functional biological probes.117–119 However, studies focusing on Cu nanoclusters for biological or chemical sensing have been rare to date. The context of base pairs in the dsDNA host greatly influences the Cu nanocluster environment and photo-luminescent properties. Label-free fluorescent CuNCs were synthesized for a rapid, simple “mix-and-measure” assay for detection of mismatch type in a DNA duplex without difficult probe DNA design and rigorously controlled temperature.73 A novel label-free, rapid, cost-effective, and highly sensitive fluorometric sensor was constructed for the detection of acetylcholinesterase (AChE) activity and its inhibitor based on the fluorescence quenching of DNA-templated copper/silver nanoclusters (DNA–Cu/AgNCs).121 A simple strategy for the preparation of strongly fluorescent and stable DNA–Cu/AgNCs from the reduction of AgNO3 and Cu(NO3)2 by NaBH4 in the presence of DNA with the sequence 50-CCCTTAATCCCC-30 has been demonstrated. The interactions among DNA with the Ag and Cu atoms are further supported by the low-temperature fluorescence data. In the presence of Cu2+ ions, the reaction time is 1.5 h, which is much shorter than that (120 h) for the preparation of Ag–DNA NCs in a mixture of AgNO3, NaBH4 and DNA without Cu2+ ions. CuNCs are commonly scaffolded by double-stranded DNA (dsDNA) and formed through the reduction of Cu2+ ions by ascorbate. CuNCs were formed in the presence of dsDNA at a low concentration of CuSO4. Their sizes were proportional to the number of base pairs in the dsDNA template and they were not formed in the case of ssDNA. Recently, fluorescent CuNCs have been synthesized with ssDNA as the scaffold. By comparing various DNA structures, such as homopolymer DNA, hairpin DNA and pristine DNA, they concluded that the thymine base in an ssDNA template played a dominant role in the formation of fluorescent CuNCs.122–125 However, the selective mechanism of CuNC growth is still unclear.4. Applications of DNA-hosted metal nanoclusters (NCs)
DNA-hosted metal NCs show great promise as optical and electrical reporters for sensitive biological and chemical detection. Because of their ultrasmall size, these metal NCs possess discrete molecule-like electronic, optical, and electrochemical properties, as well as their high specific surface area and the high loading capacity for the receptor molecules.53,54,120,126 Furthermore, they possess good water-solubility, low toxicity and high emission rates due to their subnanometer size. In the following section, recent advances in the application of DNA hosted metal NCs are highlighted.4.1 Detection of metal ions
Heavy metal ions are widespread and severe environmental pollutants that exist in different forms of metal, inorganic salts and organic complexes, which are a threat to human health. Traditional and reliable methods for the detection of metal ions have been reported based on atomic absorption/emission spectrometry and redox potentials. However, most of them are time-consuming and require large equipment. Recently, great efforts have been made towards detection of heavy metal ions in environmental and biological systems using fluorescent metal NCs as novel sensing probes. Fluorescence quenching or enhancing of metal NCs by metal ions is a direct and easy way for the detection of small molecules and metal ions, such as mercury (Hg2+), copper (Cu2+), sulfide anions (S2−) and silver ions (Ag+).53,54,66,67,127–136Hg2+ is a highly toxic and widespread pollutant ion, and its damaging effects to the brain, nervous system and kidney even at very low concentrations are well known. Wang et al. reported a highly selective and sensitive label-free method for detection of Hg2+ ions in aqueous solution by using DNA-based fluorescent Ag nanoclusters (AgNCs).132,133 The selectivity of the label-free method using DNA molecular machine-based fluorescent AgNCs was evaluated for the determination of other metal ions, including Cd2+, Zn2+, Ca2+, Fe2+, Cu2+, and Cr3+ instead of Hg2+ ions, in solution and it showed great selectivity towards Hg2+ ions. The highest fluorescence was observed in the case of Hg2+ as compared to other ions. Furthermore, this method was applied to real water samples and the results were satisfactory, suggesting that the method was reliable and practical. Dong et al. also developed a label-free method for the highly selective and sensitive detection of aqueous Hg2+ using DNA duplex stabilized AgNCs as fluorescent probes.134 The proposed detection protocol provided high sensitivity with a detection limit of 10 nM and a wider linear range for Hg2+ ions over other metal ions.
Liu et al. reported DNA-stabilized AgNCs for ratiometric and visual detection of Hg2+. By varying the DNA loop size, the sensitivity of AgNCs to Hg2+ can be precisely tuned as different emission colors can be obtained by varying the DNA sequence.135 Recently, Cao et al. developed a new fluorescent oligonucleotide-stabilized silver nanocluster (DNA/AgNCs) probe for sensitive detection of mercury and copper ions.136 The designed probe contains two tailored DNA sequences: one is a signal probe containing a cytosine-rich sequence template for AgNCs synthesis and the other is a guanine-rich sequence for signal enhancement. As shown in Fig. 7, AgNCs was firstly synthesized using a cytosine-rich single stranded DNA, and then a guanine-rich sequence was hybridized with the DNA/AgNCs for signal enhancement. The effect of the number of metal ions on DNA/AgNCs emission were tested and it was found that only two metals, Hg2+ and Cu2+, could effectively quench the emission of both as-prepared DNA/AgNCs. The experiments showed that mercury and copper ions in the range of 6.0–160.0 nM and 6–240 nM can be linearly detected with detection limits of 2.1 and 3.4 nM, respectively. The analytical parameters of the method for mercury and copper ions detection are better than those obtained using single-strand DNA/AgNCs.
 | ||
| Fig. 7 Guanine-rich sequence enhances the fluorescence of the DNA/Ag nanocluster upon detection of Hg2+ or Cu2+. (This figure has been reproduced from ref. 136 with permission from Elsevier.) | ||
4.2 Detection of nucleic acids
Development of highly sensitive and selective detection methods for nucleic acids (e.g., DNA and RNA) is beneficial to pathogen identification, clinical diagnosis, and forensic analysis. In recent years, DNA-stabilized metal NCs have played an important role in nucleic acid detection, such as mutant DNA, micro RNAs, single polynucleotide mutation and single polynucleotide polymorphism.137–139Wang et al. developed DNA-templated silver NCs for electrochemical detection of DNA hybridization.140 The method showed high sensitivity of electrochemical stripping transduction with an effective discrimination against nonhybridized DNA. Such attractive performance was illustrated for the detection of DNA segments related to the BRCA1 breast cancer gene. Werner and his colleagues reported a DNA–silver NC probe (NanoCluster Beacon, NCB) that “lights up” upon target binding.141 The developed method shows the NCB detection of an influenza target with an S/B ratio of 175, a factor of 5 better than the conventional molecular beacon probe. Since the observed fluorescence enhancement is caused by intrinsic nucleobases, their detection technique is simple, inexpensive, and compatible with commercial DNA synthesizers. Fig. 8 shows the schematic and data of a NCB for specific DNA detection.
 | ||
| Fig. 8 (a) Schematic representation of NCB probe design. (b) Quantitative and specific detection of DNA target. Kras sequence was used as nonspecific control while Braf was the sample target. (c) and (d) Fluorescence images of Braf sample target and without target. (e) The S/B ratio (red bar, left axis), target-specific signal (Itarget–Ibuffer, light blue bar, right axis), and background fluorescence (Ino target–Ibuffer, dark blue bar, right axis) from the second NCB design (NCB_2) and a molecular beacon compared for influenza target detection. (This figure has been reproduced from ref. 141 with permission from the American Chemical Society.) | ||
Fang et al. developed cadmium sulfide nanocluster-based electrochemical stripping detection of DNA hybridization.142 As a result, only a complementary sequence showed an electrochemical response as it forms a double-stranded dsDNA–CdS with the DNA probe. A negligible response was observed with a three-base mismatch sequence and a non-complementary sequence. The combination of the large number of cadmium ions released from each dsDNA hybrid with the remarkable sensitivity of the electrochemical stripping analysis allows detection at levels as low as 0.2 pmol L−1 of the complementary sequence of DNA. Willner et al. reported two types of nucleic-acid-stabilized silver nanoclusters (AgNCs) for the multiplexed analysis of a series of genes of infectious pathogens. One type includes the red-emitting AgNCs (616 nm) and the second type is near infrared-emitting AgNCs (775 nm), which was implemented for the analysis of hepatitis B virus gene (HBV), the immunodeficiency virus gene (HIV), and the syphilis (Treponema pallidum) gene.143 Wang et al. developed a novel biosensor that leads to photoinduced electrons between DNA/Ag fluorescent nanoclusters (NCs) and G-quadruplex/hemin complexes for sensitive and selective detection of DNA and ATP.144 The method is very simple and does not require separation and labeling.
Recently, Zhang et al. developed a DNA–silver nanocluster for label-free detection of DNA. The method was applied to detect H1N1 target DNA.145 Ye et al. reported label-free detection of sequence-specific DNA based on surface plasmon enhanced energy transfer (SPEET) between a fluorescent DNA/AgNC string and gold nanoparticles (AuNPs).146 In the presence of target DNA, the sensing probe binds with target DNA to form duplex DNA, which leads to a salt-induced AuNP aggregation and subsequently a weakened SPEET process between the fluorescent DNA/AgNC string and the AuNPs. The proposed method achieved a low detection limit with a simple design, convenient operation, and low experimental cost because no chemical modifications, organic dyes, enzymatic reactions, or separation procedures were involved. Hosseini et al. developed DNA based silver NCs for label free colorimetric and fluorimetric detection of methylated DNA for early diagnosis of cancer.147 The optical and fluorescent spectral behaviors were highly reproducible and clearly differentiated between unmethylated, methylated and even partially methylated DNA in CpG rich sequences. The method was also applied to human plasma samples and showed similar reproducible results.
In recent years, DNA based silver NCs have been reported for detection of micro RNAs (miRNAs).148–151 miRNAs are abundant in many human cell types as well as regulating multiple genes associated with human cancer, neurological diseases, and viral infections. Methods such as northern blotting, quantitative real-time PCR (qRT-PCR), and microarray-based hybridization are widely used for analyzing miRNAs. However, these methods have several limitations, such as poor reproducibility with interference from cross-hybridization, low selectivity and sensitivity, time-consuming, or large amounts of sample required. Thus, DNA based metal NCs can play a vital role in detection of miRNAs for early diagnosis. Vosch and his colleague developed a silver nanocluster DNA probe for the rapid detection of miRNAs. The method was limited to plant RNA-miR160 detection.148
Ye et al. reported a DNA-scaffold AgNCs probe based on isothermal amplification for the detection of miRNAs at the attomolar concentration level.149 As shown in Fig. 9(a), the unimolecular template involves five regions: two A sequences, 2 X sequences and a B sequence. Sequence A is complementary to target miRNAs while B is complementary to reporter oligonucleotide R, which acts as a scaffold for the synthesis of fluorescent AgNCs. The reaction is initiated by hybridization of A with the target miRNA T (as a trigger). The formation of a partial duplex can be extended in the presence of polymerase/dNTPs, which results in a stable double-stranded DNA duplex with two recognition sites X for the nicking enzyme Nt·BstNBI. The cleavage of the first recognition site results in the initiation of a secondary polymerization cycle while displacing the sequence T′ complementary to A, leading to exponential amplification of the trigger. The sequence of T′ was the same as that of the target miRNA T except for the change of U to T and the change of ribonucleotides to deoxyribonucleotides. In addition, the cleavage of the second recognition site can produce the reporter oligonucleotide R through a polymerization and displacement reaction cycle. Fig. 9(b) shows the specificity of miRNAs. The miR-141 belongs to the miR-200 family, which contains five members, namely miR-200a, miR-200b, miR-200c, miR-141, and miR-429. Three members of the miR-200 family (miR-141, miR-200b, and miR-429), miR-21, and let-7d were used for the evaluation of the sequence specificity of the AgNC-based assay. Specificity in the work was well addressed when the target miRNA was present in very low concentrations compared to nontarget miRNAs.149
 | ||
| Fig. 9 (a) Schematic representation of working DNA scaffold silver nanocluster probe. (b) Specificity towards detection of miRNAs. (This figure has been reproduced from ref. 149 with permission from the American Chemical Society.) | ||
Yuan et al. developed a DNA templated silver nanocluster for label free electrochemical detection of miRNAs.150 A cytosine (C)-rich loop DNA template was labeled with a silver nanocluster and used for signal amplification for the detection of miRNA-199a. The developed method provides a substantially amplified current response for highly sensitive detection of miRNA-199a down to 0.64 fM.
Wang et al. developed a fluorescent silver nanocluster for identification of single nucleotide mutations.152 Cytosine loops were inserted into probe DNA strands, which hybridized with target strands to form duplex DNA scaffolds for the generation of fluorescent AgNCs. The formation of fluorescent AgNCs in these duplex scaffolds was highly sequence-dependent and they were able to specifically identify a typical single-nucleotide mutation for sickle cell anemia mutation. Wang and his group also reported DNA hosted copper nanoclusters for identification of single nucleotide polymorphisms.153 This method successfully distinguished match and mismatch sequences with 15-mer probe DNA in solution at room temperature and demonstrated that the method is rapid and reliable.
4.3 Detection of small biomolecules
Various biomolecules, such as biothiols, proteins and enzymes, play important roles in our biological system. Abnormal alteration levels of these biomolecules may indicate some features of pathological conditions, such as diabetes, Parkinson's disease, Alzheimer's disease and many more.127–131 In recent years, DNA based AgNCs have been reported for detection of biomolecules such as lysozymes,154 thiols,155 endonucleases,156 adenosine and adenosine deaminase,157 human α-thrombin,158 cholesterol,159 cysteine,160 hydrogen peroxide and glucose.161 DNA hosted silver/copper NCs have been developed for the detection of single stranded DNA binding protein,162 histidine,163 acetylcholine esterase activity164 and a-fetoprotein.165 Silver/gold NCs based on DNA also been reported for detection of deoxyribonuclease-I.166Shao et al. developed a DNA templated silver NCs probe with photoluminescence properties and sensing ability for lysozymes. Studies showed that the DNA bases, sequence, and secondary structure could affect the photoluminescence and sensing properties of the AgNCs probe.154 Qu et al. reported DNA–AgNCs for detection of thiol compounds based on fluorescence turn on phenomena.155 Chu et al. developed a DNA–AgNCs probe for label-free detection of endonuclease activity by monitoring variations in fluorescence intensity. The study showed that significant enhancement in fluorescence was observed in the presence of endonucleases.156 A label-free fluorescent probe based on DNA-templated AgNCs was reported by Ye and co-workers for the detection of adenosine and adenosine deaminase. A guanine-rich (G-rich) DNA sequence was used as a signal transducer.157 Chu et al. developed a label free and turn on fluorescence probe based on DNA Ag-NCs for the detection of hydrogen peroxide and glucose.161 The developed probe works on a similar phenomenon to that used by Ye and his team with a guanine-rich (G-rich) DNA sequence as a signal transducer. Guanine rich DNA sequence based AgNCs probes were also reported for the detection of human α-thrombine158 and cholesterol.159 As shown in Fig. 10(A), the DNA AgNC probe for the detection of human α-thrombin consists of 15 aptamers modified by adding a stem sequence (6–30 nucleotides) and an AgNCs nucleation sequence (12 nucleotides) at the 5′-end, and Apt29 was modified by adding a complementary stem sequence (6–30 nt) and a G-rich overhang (18 nt) at the 3′-end. Fig. 10(B) shows the effect of the length of the stem sequences at the excitation wavelength of 581 nm. The 12 nucleotide hybridization sequence produced the best signal-to-background.
 | ||
| Fig. 10 (A) Schematic representation of DNA based AgNC probe for detection of human α-thrombin. (B) Effect of the length of stem sequences on the signal-to background ratio for detecting human α-thrombin (concentration of human α-thrombin was 50 nM). (This figure has been reproduced from ref. 158 with permission from the American Chemical Society.) | ||
Li et al. developed a DNA functionalized AgNC chemopalette for the detection of cysteine. They used a cytosine-rich (C-rich) single stranded oligonucleotide (ssDNA) as a template to develop a highly selective and sensitive on–off sensor for the detection of cysteine. To test the selectivity developed sensor, 19 kinds of a-amino acids, cysteine and biothiols such as GSH and Hcy were investigated under optimal conditions. The change in the fluorescence intensity of the DNA–AgNCs in the presence of cysteine was larger than those of other amino acids.160 A DNA based silver/copper NC probe for the detection of single stranded binding protein (SSB) was developed by Chang et al. Studies showed that the detection of SSB was based on a strong and specific interaction with DNA–Cu2+/AuNCs, resulting in a change in the confirmation of the DNA template. This leads to a decrease in interaction with the DNA–Cu2+/AuNCs probe and thus a decrease in the fluorescence intensity.162 Shi et al. reported a DNA based silver/copper NC probe for the detection of histidine. The fluorescence of DNA–AgNCs was greatly quenched with the addition of Cu2+. Copper shows a strong interaction with histidine and thus the DNA–AgNC/Cu2+ probe exhibited significant fluorescent enhancement in the presence of histidine, which showed the possibility of constructing a turn-on chemosensor for histidine.163 Yao et al. developed a DNA–Cu/AgNC probe for the detection of acetylcholinesterase (AChE) activity and its inhibitor based on fluorescence quenching. In this study, AChE catalyzes the hydrolysis of acetylthiocholine (ATCh) to form thiocholine, which induces fluorescence quenching of DNA–Cu/AgNCs.164 Recently, He et al. reported a DNA–Cu/AgNC probe for the detection of a-fetoprotein (AFP) in human blood serum. The detection range for AFP was 10 ng mL−1 to 150 ng mL−1 and the detection limit was 4 ng mL−1 (0.042 nM).118
Yang et al. developed a DNA based gold/silver NC probe for the detection of deoxyribonuclease-I (DNase I). The method was based on quenching the fluorescence of DNA–Au/AgNCs by DNase I digestion of the DNA (5′-CCCTTAATCCCC-3′) template.166 Fig. 11(A) shows the schematic illustration of the developed DNA–Au/Ag NC sensor. The DNA–Au/Ag NC sensor was developed by reduction of chloroauric acid and silver nitrate with sodium borohydride. Fig. 11(B) shows the fluorescence emission spectra of the DNA–Au/AgNCs sensor in the presence of DNase I at various concentrations. As shown in Fig. 11(C), the particle size of the DNA–Au/AgNCs sensor was greatly increased in the presence of DNase I. The size of DNA–Au/AgNCs was 1 to 3 nm in the absence of DNase I while in the presence of DNase I, agglomeration is observed and a size of 80 to 95 nm was observed. The method depends on enzymolysis of the DNA–Au/AgNCs templates by DNase I, possibly leading to aggregation of the Au/AgNCs.167
 | ||
| Fig. 11 (A) Schematic representation of development of DNA–Au/Ag NC sensor, which shows quenching upon interaction with DNase I. (B) Fluorescence spectra of DNA–Au/Ag NC sensor in the presence of DNase I at different concentrations (from bottom: 60 μg mL−1, 13 μg mL−1, 6 μg mL−1, 1.3 μg mL−1, 600 ng mL−1, 130 ng mL−1, 60 ng mL−1, 13 ng mL−1, and 0 ng mL−1). (C) Particle size distribution in the absence and presence of DNase I. (This figure has been reproduced from ref. 166 with permission from Elsevier.) | ||
4.4 Detection of toxins
Ochratoxin A (OTA) is extremely important for food safety since it is one of the most toxic and widespread mycotoxins. Zhang et al. developed a fluorescent aptasensor based on a DNA scaffold AgNC for the detection of OTA I in real samples of wheat.167 The aptasensor for the detection of OTA consists of a biotin group functionalized with aptamer Ap (purple color) and attached to streptavidin-modified magnetic beads, as shown in Fig. 12(A). The cytosine rich single strand Sp (black color) is partly complementary to Ap and was added to form the Ap–Sp duplex structure. The stability of the G-quadruplex is higher than that of the Ap–Sp duplexes, so in the presence of OTA, Ap can bind with OTA to form a G-quadruplex while releasing the single-strand Sp. On magnetic separation, the released Sp was left in the supernatant liquid. Upon the addition of Ag+, the Sp could act as a scaffold for the synthesis of fluorescent silver nanoclusters (AgNCs) through the reduction of NaBH4 owing to the high affinity of Ag+ for the cytosines of Sp. The resulting Sp-scaffolded AgNCs exhibited excellent fluorescence emission at 632 nm upon excitation at 574 nm. However, in the absence of OTA, the Ap–Sp modified MBs were separated by a neodymium magnet placed under the centrifuge tube. Therefore, the supernatant liquid contained no single-strand Sp and could not react with Ag+ to synthesize AgNCs. Thus, this method offers a simple, highly sensitive, selective and green chemical sensing platform for the detection of OTA [120. A detection limit as low as 2 pg mL−1 was found for OTA detection with good linearity, as shown in Fig. 12(B) and (C). The method was also applied for the detection of other mycotoxins such as OTB, AFB1, and FB, and the results showed that OTA has greater affinity than the other mycotoxins.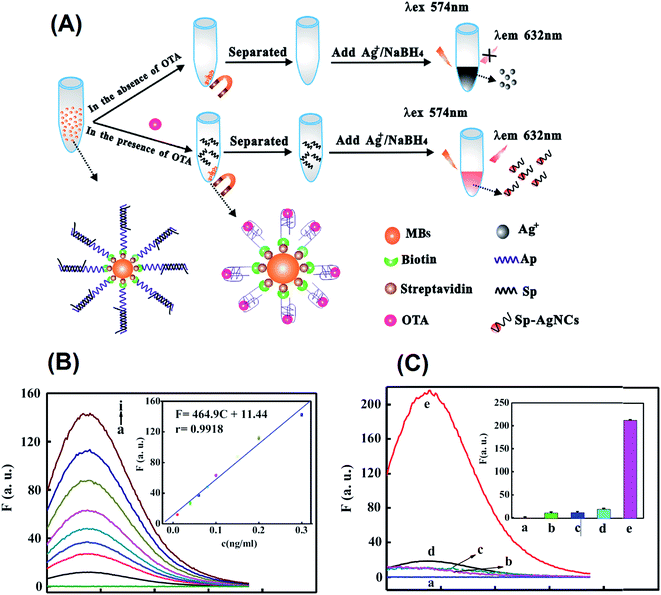 | ||
| Fig. 12 (A) Principle of developed aptasensor for detection of OTA. (B) Calibration curve and fluorescence spectra of the aptasensor with various concentrations of OTA (from bottom to top): 0.00, 0.01, 0.04, 0.06, 0.08, 0.10, 0.15, 0.20, and 0.30 ng mL−1. (C) Selectivity of the aptasensor toward OTA against other mycotoxins at the same concentrations (1 ng mL−1). Blank (a), AFB1 (b), FB (c), OTB (d), and OTA (e). (Inset) Each data point represents the average value of three independent experiments with error bars indicated. (This figure has been reproduced from ref. 167 with permission from Elsevier.) | ||
Huang et al. reported a DNA template AgNC probe for the detection of bleomycin.168 Bleomycin is an anti-cancer drug; however, it has serious dose-limiting side effects. In this method, bleomycin coordinates with Fe2+, which can highly oxidize the core of fluorescent silver nanoclusters. Very little fluorescence intensity change of DNA–AgNCs was observed when either bleomycin or Fe2+ was added. On addition of a mixture of bleomycin and Fe2+, the fluorescence was quenched significantly. The significant influence of the complex of bleomycin and Fe2+ on the fluorescence of the DNA–AgNC probe indicates that there is a strong interaction between them and thus the developed method can detect of bleomycin.
4.5 Detection of explosives
The rapid and sensitive detection of nitroaromatic explosives is of great interest these days for safety concerns. Recently, Willner et al. developed a DNA based AgNCs hybrid for the detection of explosives such as picric acid, trinitrotoluene (TNT), and hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX).169 Fig. 13 illustrates the analysis of explosives by AgNCs. The nucleic acid (1) acts as a scaffold for organizing the various components of the system. It contains two hybridization domains for nucleic acids (2) and (3). The nucleic acid (2) includes two domains, I and II, where domain I stabilizes the AgNCs (λex = 520 nm, λem = 615 nm), and domain II hybridizes with the complementary domain of the scaffold (1). The amino-modified nucleic acid (3) is functionalized with the π-donor units, L-DOPA (4), L-tyrosine (5), or 6-hydroxy-L-DOPA (6), which hybridizes with the complementary domain of the scaffold (1). In the presence of nitro-substituted explosives, the nitro-substituted electron-acceptor substrates bind to the π-donor sites via donor–acceptor interactions. This leads to the electron-transfer quenching of the luminescence of the AgNCs. The sensitivities of the analytical platforms are controlled by the electron-donating properties of the donor substituents, and 6-hydroxy-L-DOPA was found to be the most sensitive donor.169–171 | ||
| Fig. 13 Schematic illustration of DNA based AgNCs hybrid for the detection of explosives picric acid, TNT and RDX. The quenching of the luminescence of the AgNCs by the explosive substrates via donor–acceptor interactions provides the readout signal. (This figure has been reproduced from ref. 169 with permission from the American Chemical Society.) | ||
4.6 Catalysis transformation
In recent years, DNA based metal NCs have been developed for oxygen reduction reaction activity, biocatalytic transformation, DNA methyltransferase activity and DNA detection via exonuclease III-assisted cyclic amplification based on fluorescence and electrochemical detection as a sensing platform.87,125,172–174The oxygen reduction reaction (ORR) is attracting a great deal of attention in electrochemistry owing to its technological importance in electrochemical energy conversion and storage devices, like fuel cells and secondary metal–air batteries. Additionally, the ORR is important in water electrolysis, corrosion, and diverse industrial processes. Kim et al. reported platinum (Pt) nanoclusters on a genomic DNA–graphene hybrid oxide (gDNA–GO) composite for ORR.172 In their work, electrochemical data showed that the Pt/gDNA–GO composite exhibits a greater ORR onset potential, ORR half-wave potential, specific activity and mass activity as compared with those of Pt nanoparticles/GO and Pt/C catalysts. The Ptn/gDNA–GO composite displays excellent accelerated durability (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) and long-term CV stability (10
000 cycles) and long-term CV stability (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) as compared with Pt nanoparticles/GO and Pt/C catalysts in acidic media with significant corrosion resistance and high conductivity.172 Martinez et al. developed a DNA-templated gold nanocluster for enhanced enzymatic reduction of oxygen.87 The DNA–AuNCs hybrid was integrated with bilirubin oxidase (BOD) and single walled carbon nanotubes (SWNTs). The AuNC is electrochemically active and enhances the performance of the BOD catalyzed enzymatic ORR by lowering the over potential by ∼15 mV and improving the electronic communication between the electrode and the enzyme active site. Thus, the designed system can play a critical role in biofuel cell design.87
000 cycles) as compared with Pt nanoparticles/GO and Pt/C catalysts in acidic media with significant corrosion resistance and high conductivity.172 Martinez et al. developed a DNA-templated gold nanocluster for enhanced enzymatic reduction of oxygen.87 The DNA–AuNCs hybrid was integrated with bilirubin oxidase (BOD) and single walled carbon nanotubes (SWNTs). The AuNC is electrochemically active and enhances the performance of the BOD catalyzed enzymatic ORR by lowering the over potential by ∼15 mV and improving the electronic communication between the electrode and the enzyme active site. Thus, the designed system can play a critical role in biofuel cell design.87
Willner et al. developed a luminescent DNA/silver nanocluster probe for biocatalytic transformation of enzyme activity.125 The study showed that H2O2 quenches the luminescence of the AgNCs, which enables the probing of biocatalytic oxidase-stimulated H2O2-generating biotransformations. Furthermore, this was illustrated by the example of the analysis of glucose oxidase (GOx)-mediated oxidation of glucose, which yields gluconic acid and H2O2. Furthermore, quinone derivatives quench the luminescence of the AgNCs, and this function enables the probing of tyrosinase (TYR), which is a melanoma cancer cell biomarker. The TYR-stimulated oxidation of tyrosine, dopamine, or tyramine yields quinone derivatives that quench the fluorescence of the AgNCs and enable the sensitive detection of tyrosinase.125 Zhou et al. developed a fluorescent silver nanocluster hairpin shaped DNA probe for the detection of DNA methyltransferase activity.173 The developed probe consists of a 5′-C-rich/G-rich-3′ tails sequence. Their study suggests that in the presence of DNA methyltransferase, the methylation-sensitive restriction endonuclease DpnI, which has the same recognition site as the DNA methyltransferase, can split the probe, freeing the G-rich sequence from the C-rich sequence, thus quenching the fluorescence of DNA–AgNCs. The method was also employed for detection of other three proteins, namely BSA, tyrosinase and Klenow polymerase, and was found to be selective for the detection of DNA methyltransferase.173 Ye et al. also reported a DNA scaffolded silver nanocluster molecular beacon (AgNC–LMB) for DNA detection via exonuclease III (Exo III)-assisted cyclic amplification.174 The proposed method involves two processes: target-mediated digestion by Exo III and the synthesis of AgNC–LMB as a switch for the signal output. Upon hybridization of the rationally designed probe with the target, Exo III removes nucleotides from the 30 terminus of the probe, and the resultant fragment leads to the fluorescence enhancement of the AgNCs.174
4.7 Device circuit development
Molecular computing holds great promise for applications in computer and life sciences. It is interesting to design and construct versatile logic devices with the ability to perform multiple operations, which would meet the need of future development of DNA circuits. With this consideration, Wang et al. developed DNA/Ag fluorescent nanoclusters as a versatile logic device for K+ and H+ as two inputs.175 Hairpin DNA (HP26) with a poly-C loop serves as the template for synthesizing two species of Ag nanoclusters. As shown in Fig. 14(A), HP26 consists of G-rich, poly-C, and C-rich regions. Upon input of K+ and H+, HP26 is able to convert into the G-quadruplex (G4) and/or i-motif structures, and different species of Ag nanoclusters have distinct fluorescence responses to the inputs. This enables two or more logic operations to be performed together via a multichannel fluorescence output. Fig. 14(B) shows the fluorescence emission spectra of HP26-stabilized Ag nanoclusters for multiple logic operations. | ||
| Fig. 14 (A) Schematic representation of logic operation of HP26/Ag nanocluster. (B) HP26-stabilized Ag nanoclusters for multiple logic operations. (a–c) Fluorescence emission spectra of Ag nanoclusters at different excitation wavelengths in 10 mM Tris–Ac buffer (pH 8.0) at four input modes: no input 1; 100 mM K+ 2; H+ (pH 5.0) 3; K+ and H+ 4. (d–f) Bar representations of the fluorescence intensity at 570 nm (FI570), 646 nm (FI646), and 601 nm (FI601). The same threshold value (0.4) for output 1 or 0 is set at all fluorescence channels. (C) Logic operations as NOR, NOT and AND gates. (This figure has been reproduced from ref. 175 with permission from the American Chemical Society.) | ||
As shown in Fig. 14(B), when the HP26/Ag NC probe is excited at 494 nm, the yellow fluorescence emitter becomes active. In the absence of H+, the fluorescence is strong (output 1) while the signal is relatively low (output 0) upon input of H+. This represents NOT logic gate behavior, which is shown more clearly by a bar presentation of the fluorescence output in Fig. 14(B). The red emitter becomes active upon changing the excitation wavelength to 581 nm (Fig. 14(B)). Its fluorescence emission is observed without any input, while addition of K+ and/or H+ sharply lowers the fluorescence signal. The corresponding bar presentation shown in Fig. 14(B) is consistent with a two-input NOR gate behavior. If the excitation wavelength is changed to 537 nm, a strong orange emission is observed in the presence of both K+ and H+. This is concordant with a two-input AND gate behavior. The true behaviors of the NOT, NOR and AND logic gates are shown in Fig. 14(C).175 The developed device has great potential and application towards further development of DNA circuits.
4.8 Biological labeling and imaging
Optical imaging is an emerging technology with great potential for improving disease prevention, diagnosis and treatment in medical science. Owing to its simplicity and visualization, optical imaging has been an area of great interest. DNA-based metal NC offer great potential in biological labeling owing to their tiny size, biocompatibility, sensitivity, multiplex detection capabilities and equipment cost.5–7,48–51,127–131Dickson et al. developed DNA based AgNCs for intracellular staining,176 transfection of living cells177,178 and live cell surface labeling.179 Dickson's group showed that the developed AgNCs can directly transferred from a low-molecular-weight poly(acrylic acid) shuttle to a single-stranded DNA tag on the protein of interest. Upon transfer, the fluorescence of the DNA–AgNCs increases more than ten times, which provides bright and photostable labeling.177 Similarly, Dickson et al. reported fluorescent AgNCs encapsulated by single stranded oligo-DNA (24 cytosine base pairs) to monitor transfected HeLa cells. They used the transfection reagent lipofectamine to facilitate the internalization of the DNA–AgNCs into living HeLa cells.178 In another study by Dickson's group, they developed DNA (24mer cytosine) encapsulated Ag–NCs (C24–AgNCs) conjugated to proteins and observed when staining with a live cell surface. The biotinylated fixed NIH-3T3 cell showed much higher fluorescence than the non-biotinylated cells after incubation with 5 mM avidin conjugated C24–AgNCs. When using penetratin conjugated C12–AgNCs, stronger staining of nuclei compared to other organelles in NIH-3T3 cell was observed.179
Wang et al. developed a label free and turn on aptamer strategy for the detection of cancer cells. They developed a DNA–AgNCs based aptamer that gives high fluorescence upon conformation alteration of the aptamer in the presence of a cancer cell line.180 In their strategy, two types of DNA probe were recognized. One aptamer consists of a hairpin shaped target specific sequence at the 3′-end, a guanine-rich DNA sequence named the S-Probe, and an arm segment at the 5′-end (denoted as the recognition probe) named the R-Probe. The other, serving as a signal probe, contains a sequence for AgNCs templated synthesis and a link sequence complementary to the arm segment of the recognition probe. As shown in Fig. 11, when the DNA–AgNCs based aptamer binds to cancer cells, it enforces the recognition probe to undergo a conformational alteration and then initiates hybridization between the arm segment of the recognition probe and the link sequence of the signal probe. The AgNCs are then close to the guanine-rich DNA, leading to an enhanced fluorescence readout. As proof-of-concept, detection of CCRF-CEM cancer cells and Ramos cells was performed by using the specific aptamer, sgc8c, which was identified to interact with cell membrane protein tyrosine kinase-7. Ramos cells were used as control cells. Two control probes named CR-Probe 1, which is the R-Probe without the guanine-rich DNA sequences, and CR-Probe 2 which is the R-Probe with the sgc8c sequence changed to a random DNA sequence, were used. Fig. 15(B)[i] shows the confocal laser scanning microscopy images of the CCRF-CEM cells and the Ramos cells. The image analysis showed that only CCRF-CEM cells show fluorescence. However, no fluorescence was seen in the control groups. Additionally, this strategy for target cell detection was also tested using flow cytometry (Fig. 15(B)[ii]). The same trend of selective binding to CCRF-CEM cancer cells was observed. The general applicability of the strategy is also achieved in the successful detection of Ramos cells using the TD05 probe.180
 | ||
| Fig. 15 (A) Schematic illustration of turn on aptamer strategy for detection of cancer cells by DNA–AgNCs upon hybridization. (B) Confocal laser scanning microscopy images of CCRF-CEM cells or Ramos cells incubated with various probes. In (i), (a–d) are the fluorescence images of CCRF-CEM cells incubated with the R-Probe and S-Probe-AgNCs, Ramos cells incubated with the R-Probes and S-Probes-AgNCs, CCRF-CEM cells incubated with the CR-Probes 1 and S-Probes-AgNCs, and CCRF-CEM cells incubated with the CR-Probes 2 and S-Probes-AgNCs, respectively. The inserted image in (a) is the magnified image of the labeled CCRF-CEM cell with the R-Probe and S-Probe-AgNCs (scale bar is 10 μm). The images of (a′–d′) are the corresponding merged images. (ii) Corresponding flow cytometry analysis of target CCRF-CEM cells or non-target Ramos cells incubated with different probes. (This figure has been reproduced from ref. 180 with permission from the American Chemical Society.) | ||
In a study by Zhu et al., nuclei of MCF-7 human breast-cancer cells were found to be specifically stained with red color when interacting with aptamer functionalized AgNCs. The DNA scaffold was composed of AS1411 aptamer, poly(cytosine) and a –TTTTT– loop connecter.181 Gao and co-workers designed a AgNC–aptamer hybrid that can target the nucleus of live cells of CCRF-CEM. Eight cytosine bases were inserted at the 5′ end of the sgc8c aptamer and named as Msgc8 aptamer.182 Recently, Li et al. reported a green staining method for DNA in polyacrylamide gel electrophoresis based on fluorescent copper nanocluster (CuNCs). In their study, DNA-templated CuNCs were able to make the DNA band visible under UV light. In animal studies, the developed CuNCs were found to be non toxic in contact with skin.126
5. Summary and future perspective
Briefly, we have discussed the recent progress with DNA hosted metal NCs and their sensing applications in biomedical and environmental science. Researchers need to simultaneously tackle a number of challenges to bring the novel metal NC research to the next level, such as monodispersed and site-specific synthesis, theoretical modeling, structure determination, and single molecule characterization of metal NCs. A significant effort has been made to address the detection of metal ions, nucleic acids and small biomolecules, but future work should target logic circuit development, detection of explosives/toxicants and cell imaging. Still, the application of metal nanoclusters in sequence recognition has shown great promise in achieving high selectivity, which is difficult to accomplish with conventional methods. By synthesizing smart probe metal nanoclusters, the rapid, reliable “mix-and-measure” detection of mismatch type can be achieved with different probe DNA in solution, which opens new opportunities for research in DNA based diagnostics. Most of the applications of metal NCs for fluorescence imaging have focused on in vitro systems, such as cultured cells, with very limited work in vivo. Fluorescent metal NCs can be employed with other molecular imaging modalities, such as magnetic resonance imaging (MRI), positron emission tomography (PET), and computed tomography (CT). Such combinations may evolve into powerful techniques for diagnostic research and applications.DNA based AgNCs have been widely employed for various applications owing to their fluorescence properties. However, they have some disadvantages, such as toxicity, unstable under critical conditions (e.g., salt and high temperature), high cost compared to other NCs, and purification issues. Studies are needed with other metal NCs, such as Au, Cu, Mg, bimetallic NC, polymeric NC and many others, which could overcome these problems. Besides, DNA templated metal NCs with metals such as Pt and Au can also be synthesized, which could offer excellent electrochemical properties. Hence, they can play a pivotal role in the development of DNA based sensing devices based on electrochemical detection.
The next generation could interface such NCs with novel types of materials, such as DNA origami, graphene, carbon nanotubes and hydrogels, to name a few. Evidently, DNA-templated NCs have emerged as model engineered nanomaterials that may provide leads for future development and application of novel and ‘safe by design’ nanomaterials. The long-term goal of this research area is to explore novel, promising, environmentally benign approaches to construct switchable nanomachines, nano/subnano clusters and enantioselective recognition platforms through DNA-based conjugation and modulation.
Acknowledgements
This work was supported by the SERB, India under Young Scientist Start-up Grant (Project Code: YSS/2015/000258).References
- L. Zhang and E. Wang, Nano Today, 2014, 9, 132–157 CrossRef CAS.
- J. Zheng, P. Nicovich and R. Dickson, Annu. Rev. Phys. Chem., 2007, 58, 409–431 CrossRef CAS PubMed.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116(18), 10346–10413 CrossRef CAS PubMed.
- G. Dharanivasan, S. Mohammed Riyaz, D. Michael Immanuel Jesse, T. Raja Muthuramalingam, G. Rajendran and K. Kathiravan, RSC Adv., 2016, 6, 11773–11785 RSC.
- B. Han and E. Wang, Anal. Bioanal. Chem., 2011, 402, 129–138 CrossRef PubMed.
- N. Goswami, Q. Yao, Z. Luo, J. Li, T. Chen and J. Xie, J. Phys. Chem. Lett., 2016, 7, 962–975 CrossRef CAS PubMed.
- A. Pandya, P. Sutaria, A. Lodha, H. Goswami and S. Menon, Mol. Cytogenet., 2014, 7, P80 CrossRef.
- A. Latorre and Á. Somoza, ChemBioChem, 2012, 13, 951–958 CrossRef CAS PubMed.
- Q. Yuan, Y. Wang, L. Zhao, R. Liu, F. Gao, L. Gao and X. Gao, Nanoscale, 2016, 8, 12095–12104 RSC.
- S. Choi, R. Dickson and J. Yu, Chem. Soc. Rev., 2012, 41, 1867–1891 RSC.
- D. Pichugina, N. Kuz'menko and A. Shestakov, Russ. Chem. Rev., 2015, 84, 1114–1144 CrossRef CAS.
- G. Schmid and D. Fenske, Philos. Trans. R. Soc., A, 2010, 368, 1207–1210 CrossRef CAS PubMed.
- Y. Sun, Science, 2002, 298, 2176–2179 CrossRef CAS PubMed.
- H. He, Y. Cheng, C. Yang, G. Zeng, C. Zhu and Z. Yan, J. Environ. Sci., 2016 DOI:10.1016/j.jes.2016.06.009.
- A. Pandya, P. G. Sutariya, A. Lodha and S. K. Menon, Nanoscale, 2013, 5, 2364–2371 RSC.
- A. Pandya, P. G. Sutariya and S. K. Menon, Analyst, 2013, 138, 2483–2490 RSC.
- J. Zheng and R. Dickson, J. Am. Chem. Soc., 2002, 124, 13982–13983 CrossRef CAS PubMed.
- A. Pandya, K. V. Joshi, P. G. Sutariya and S. K. Menon, Anal. Methods, 2012, 4, 3102–3106 RSC.
- A. Lad, A. Pandya and Y. Agrawal, TrAC, Trends Anal. Chem., 2016, 80, 458–470 CrossRef CAS.
- A. Lodha, A. Pandya, P. Sutariya and S. Menon, RSC Adv., 2014, 4, 50443–50448 RSC.
- A. Lodha, A. Pandya, P. Sutariya and S. Menon, Analyst, 2013, 138, 5411 RSC.
- J. Zheng, C. Zhang and R. Dickson, Phys. Rev. Lett., 2004, 93, 077402 CrossRef PubMed.
- J. Xie, Y. Zheng and J. Ying, J. Am. Chem. Soc., 2009, 131, 888–889 CrossRef CAS PubMed.
- J. Petty, J. Zheng, N. Hud and R. Dickson, J. Am. Chem. Soc., 2004, 126, 5207–5212 CrossRef CAS PubMed.
- P. Sutariya, A. Pandya, A. Lodha and S. Menon, Talanta, 2016, 147, 590–597 CrossRef CAS PubMed.
- Z. Luo, X. Yuan, Y. Yu, Q. Zhang, D. Leong, J. Lee and J. Xie, J. Am. Chem. Soc., 2012, 134, 16662–16670 CrossRef CAS PubMed.
- C. J. Lin, C. Lee, J. Hsieh, H. Wang, J. K. Li, J. Shen, W. Chan, H. Yeh and W. H. Chang, J. Med. Biol. Eng., 2009, 29(6), 276–283 Search PubMed.
- H. He, Y. Chen, X. Li, Y. Cheng, C. Yang and G. Zeng, Int. Biodeterior. Biodegrad., 2016 DOI:10.1016/j.ibiod.2016.10.007.
- J. Yu, S. Patel and R. Dickson, Angew. Chem., Int. Ed., 2007, 46, 2028–2030 CrossRef CAS PubMed.
- X. Hu, T. Liu, Y. Zhuang, W. Wang, Y. Li, W. Fan and Y. Huang, TrAC, Trends Anal. Chem., 2016, 77, 66–75 CrossRef CAS.
- A. Yahia-Ammar, D. Sierra, F. Mérola, N. Hildebrandt and X. Le Guével, ACS Nano, 2016, 10, 2591–2599 CrossRef CAS PubMed.
- J. Zhang, F. Cheng, J. Li, J. Zhu and Y. Lu, Nano Today, 2016, 11, 309–329 CrossRef CAS PubMed.
- N. Goswami, F. Lin, Y. Liu, D. Leong and J. Xie, Chem. Mater., 2016, 28, 4009–4016 CrossRef.
- A. Pandya, A. Tripathi, R. Purohit, S. Singh, M. Nandasiri, A. Karakoti, S. Singh and R. Shanker, RSC Adv., 2015, 5, 94236–94240 RSC.
- S. Lin, N. Chen, S. Sum, L. Lo and C. Yang, Chem. Commun., 2008, 4762 RSC.
- C. Huang, C. Chiang, Z. Lin, K. Lee and H. Chang, Anal. Chem., 2008, 80, 1497–1504 CrossRef CAS PubMed.
- J. Liu, TrAC, Trends Anal. Chem., 2014, 58, 99–111 CrossRef CAS.
- N. Navani and Y. Li, Curr. Opin. Chem. Biol., 2006, 10, 272–281 CrossRef CAS PubMed.
- W. Xu, Functional Nucleic Acids Detection in Food Safety, 2016, pp. 275–322 Search PubMed.
- P. Toren, E. Ozgur and M. Bayindir, Lab Chip, 2016, 16, 2572–2595 RSC.
- E. Cho, J. Lee and A. Ellington, Annu. Rev. Anal. Chem., 2009, 2, 241–264 CrossRef CAS PubMed.
- M. Smanski, H. Zhou, J. Claesen, B. Shen, M. Fischbach and C. Voigt, Nat. Rev. Microbiol., 2016, 14, 135–149 CrossRef CAS PubMed.
- D. Li, S. Song and C. Fan, Acc. Chem. Res., 2010, 43, 631–641 CrossRef CAS PubMed.
- I. Díez and R. Ras, Nanoscale, 2011, 3, 1963 RSC.
- J. Petty, S. Story, J. Hsiang and R. Dickson, J. Phys. Chem. Lett., 2013, 4, 1148–1155 CrossRef CAS PubMed.
- R. Jin, Nanoscale, 2010, 2, 343–362 RSC.
- H. Xu and K. Suslick, Adv. Mater., 2010, 22, 1078–1082 CrossRef CAS PubMed.
- J. Obliosca, C. Liu and H. Yeh, Nanoscale, 2013, 5, 8443 RSC.
- J. Li, Y. Dai, S. Wang, C. Han and K. Xu, Sens. Actuators, B, 2016, 232, 1–8 CrossRef CAS.
- A. Cantelli, G. Battistelli, G. Guidetti, J. Manzi, M. Di Giosia and M. Montalti, Dyes Pigm., 2016, 135, 64–79 CrossRef CAS.
- M. Sarparast, A. Noori, H. Ilkhani, S. Bathaie, M. El-Kady, L. Wang, H. Pham, K. Marsh, R. Kaner and M. Mousavi, Nano Res., 2016, 11, 3229–3246 CrossRef.
- A. Latorre, R. Lorca and Á. Somoza, J. Chem., 2013, 2013, 1–6 CrossRef.
- L. Shang, S. Dong and G. Nienhaus, Nano Today, 2011, 6, 401–418 CrossRef CAS.
- Q. Zhao, S. Chen, L. Zhang, H. Huang, Y. Zeng and F. Liu, Anal. Chim. Acta, 2014, 852, 236–243 CrossRef CAS PubMed.
- Y. Shiang, C. Huang, W. Chen, P. Chen and H. Chang, J. Mater. Chem., 2012, 22, 12972 RSC.
- J. Fan, Y. He, K. Bao, C. Wu, J. Bao, N. Schade, V. Manoharan, G. Shvets, P. Nordlander, D. Liu and F. Capasso, Nano Lett., 2011, 11, 4859–4864 CrossRef CAS PubMed.
- T. Edwardson, K. Lau, D. Bousmail, C. Serpell and H. Sleiman, Nat. Chem., 2016, 8, 162–170 CAS.
- G. Chen, I. Roy, C. Yang and P. Prasad, Chem. Rev., 2016, 116, 2826–2885 CrossRef CAS PubMed.
- C. Soto, A. Srinivasan and B. Ratna, J. Am. Chem. Soc., 2002, 124, 8508–8509 CrossRef CAS PubMed.
- A. Hung, C. Micheel, L. Bozano, L. Osterbur, G. Wallraff and J. Cha, Nat. Nanotechnol., 2009, 5, 121–126 CrossRef PubMed.
- M. Boles, M. Engel and D. Talapin, Chem. Rev., 2016, 116(18), 11220–11289 CrossRef CAS PubMed.
- D. Nykypanchuk, M. Maye, D. van der Lelie and O. Gang, Nature, 2008, 451, 549–552 CrossRef CAS PubMed.
- Z. Wu and R. Jin, Nano Lett., 2010, 10, 2568–2573 CrossRef CAS PubMed.
- J. Xie, Y. Zheng and J. Ying, J. Am. Chem. Soc., 2009, 131, 888–889 CrossRef CAS PubMed.
- D. Joseph and K. Geckeler, Colloids Surf., B, 2014, 115, 46–50 CrossRef CAS PubMed.
- J. Li, J. Zhu and K. Xu, TrAC, Trends Anal. Chem., 2014, 58, 90–98 CrossRef CAS.
- J. Zhang, F. Cheng, J. Li, J. Zhu and Y. Lu, Nano Today, 2016, 11, 309–329 CrossRef CAS PubMed.
- P. O'Neill, L. Velazquez, D. Dunn, E. Gwinn and D. Fygenson, J. Phys. Chem. C, 2009, 113, 4229–4233 Search PubMed.
- D. Schultz and E. Gwinn, Chem. Commun., 2012, 48, 5748 RSC.
- J. Sharma, R. Rocha, M. Phipps, H. Yeh, K. Balatsky, D. Vu, A. Shreve, J. Werner and J. Martinez, Nanoscale, 2012, 4, 4107 RSC.
- J. Sharma, H. Yeh, H. Yoo, J. Werner and J. Martinez, Chem. Commun., 2010, 46, 3280 RSC.
- H. Yeh, J. Sharma, J. Han, J. Martinez and J. Werner, Nano Lett., 2010, 10, 3106–3110 CrossRef CAS PubMed.
- X. Jia, J. Li, L. Han, J. Ren, X. Yang and E. Wang, ACS Nano, 2012, 6, 3311–3317 CrossRef CAS PubMed.
- R. Seidel, L. Colombi Ciacchi, M. Weigel, W. Pompe and M. Mertig, J. Phys. Chem. B, 2004, 108, 10801–10811 CrossRef CAS.
- W. Li, B. Chen, H. Zhang, Y. Sun, J. Wang, J. Zhang and Y. Fu, Biosens. Bioelectron., 2015, 66, 251–258 CrossRef CAS PubMed.
- J. Tiwari, K. Nath, S. Kumar, R. Tiwari, K. Kemp, N. Le, D. Youn, J. Lee and K. Kim, Nat. Commun., 2013, 4, 2221 Search PubMed.
- Y. Zhu, H. Qian and R. Jin, J. Mater. Chem., 2011, 21, 6793 RSC.
- A. Pinheiro, D. Han, W. Shih and H. Yan, Nat. Nanotechnol., 2011, 6, 763–772 CrossRef CAS PubMed.
- G. Dharanivasan, S. Mohammed Riyaz, D. Michael Immanuel Jesse, T. Raja Muthuramalingam, G. Rajendran and K. Kathiravan, RSC Adv., 2016, 6, 11773–11785 RSC.
- Y. Ma, H. Fu, C. Zhang, S. Cheng, J. Gao, Z. Wang, W. Jin, J. Conde and D. Cui, Sci. Rep., 2016, 6, 33436 CrossRef CAS PubMed.
- R. Zhou, M. Shi, X. Chen, M. Wang and H. Chen, Chem.–Eur. J., 2009, 15, 4944–4951 CrossRef CAS PubMed.
- S. Bhardwaj, R. Itteboina and T. Sau, ChemistrySelect, 2016, 1, 3091–3096 CrossRef CAS.
- E. Zhang, S. Xiang and A. Fu, J. Nanosci. Nanotechnol., 2016, 16, 6597–6610 CrossRef.
- B. Paramanik, D. Bain and A. Patra, J. Phys. Chem. C, 2016, 120, 17127–17135 CAS.
- T. A. C. Kennedy, J. L. MacLean and J. Liu, Chem. Commun., 2012, 48(54), 6845–6847 RSC.
- G. Liu, Y. Shao, K. Ma, Q. Cui, F. Wu and S. Xu, Gold Bull., 2012, 45, 69–74 CrossRef CAS.
- S. Chakraborty, S. Babanova, R. Rocha, A. Desireddy, K. Artyushkova, A. Boncella, P. Atanassov and J. Martinez, J. Am. Chem. Soc., 2015, 137, 11678–11687 CrossRef CAS PubMed.
- A. Lopez and J. Liu, Can. J. Chem., 2015, 93, 615–620 CrossRef CAS.
- A. West, M. Griep, D. Cole and S. Karna, Anal. Chem., 2014, 86, 7377–7382 CrossRef CAS PubMed.
- Y. Chen, G. Tao, R. Lin, X. Pei, F. Liu and N. Li, Chem.–Asian J., 2016, 11, 1677–1681 CrossRef CAS PubMed.
- Z. Hossain and F. Huq, J. Inorg. Biochem., 2002, 91, 398–404 CrossRef CAS PubMed.
- A. Ono, S. Cao, H. Togashi, M. Tashiro, T. Fujimoto, T. Machinami, S. Oda, Y. Miyake, I. Okamoto and Y. Tanaka, Chem. Commun., 2008, 4825 RSC.
- J. Wu, Y. Fu, Z. He, Y. Han, L. Zheng, J. Zhang and W. Li, J. Phys. Chem. B, 2012, 116, 1655–1665 CrossRef CAS PubMed.
- K. Park, J. Lee and H. Park, Chem. Commun., 2012, 48, 4549 RSC.
- W. Xie, W. Huang, N. Li and H. Luo, Chem. Commun., 2012, 48, 82–84 RSC.
- D. Feng, G. Liu, W. Zheng, J. Liu, T. Chen and D. Li, Chem. Commun., 2011, 47, 8557 RSC.
- T. Ihara, T. Ishii, N. Araki, A. Wilson and A. Jyo, J. Am. Chem. Soc., 2009, 131, 3826–3827 CrossRef CAS PubMed.
- T. Vosch, Y. Antoku, J. Hsiang, C. Richards, J. Gonzalez and R. Dickson, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 12616–12621 CrossRef CAS PubMed.
- M. Carro Temboury, V. Paolucci, E. Hooley, L. Latterini and T. Vosch, Analyst, 2016, 141, 123–130 RSC.
- E. Gwinn, P. O'Neill, A. Guerrero, D. Bouwmeester and D. Fygenson, Adv. Mater., 2008, 20, 279–283 CrossRef CAS.
- P. O'Neill, K. Young, D. Schiffels and D. Fygenson, Nano Lett., 2012, 12, 5464–5469 CrossRef PubMed.
- J. Li, X. Jia, D. Li, J. Ren, Y. Han, Y. Xia and E. Wang, Nanoscale, 2013, 5, 6131 RSC.
- M. Wang, G. Zhang and D. Zhang, Chem. Commun., 2015, 51, 3812–3815 RSC.
- B. Sengupta, K. Springer, J. Buckman, S. Story, O. Abe, Z. Hasan, Z. Prudowsky, S. Rudisill, N. Degtyareva and J. Petty, J. Phys. Chem. C, 2009, 113, 19518–19524 CAS.
- W. Li, L. Liu, Y. Fu, Y. Sun, J. Zhang and R. Zhang, Photochem. Photobiol. Sci., 2013, 12, 18 Search PubMed.
- S. Neidle, J. Med. Chem., 2016, 59, 5987–6011 CrossRef CAS PubMed.
- J. Ai, W. Guo, B. Li, T. Li, D. Li and E. Wang, Talanta, 2012, 88, 450–455 CrossRef CAS PubMed.
- W. Guo, J. Yuan, Q. Dong and E. Wang, J. Am. Chem. Soc., 2010, 132, 932–934 CrossRef CAS PubMed.
- Z. Huang, F. Pu, D. Hu, C. Wang, J. Ren and X. Qu, Chem.–Eur. J., 2011, 17, 3774–3780 CrossRef CAS PubMed.
- R. Orbach, W. Guo, F. Wang, O. Lioubashevski and I. Willner, Langmuir, 2013, 29, 13066–13071 CrossRef CAS PubMed.
- W. Guo, R. Orbach, I. Mironi-Harpaz, D. Seliktar and I. Willner, Small, 2013, 9, 3748–3752 CrossRef CAS PubMed.
- J. Ma, B. Yin, H. Le and B. Ye, ACS Appl. Mater. Interfaces, 2015, 7, 12856–12863 CAS.
- G. Dianov, Mutat. Res., 2003, 531, 157–163 CrossRef CAS PubMed.
- B. Saccà and C. Niemeyer, Angew. Chem., Int. Ed., 2011, 51, 58–66 CrossRef PubMed.
- S. Pal, R. Varghese, Z. Deng, Z. Zhao, A. Kumar, H. Yan and Y. Liu, Angew. Chem., Int. Ed., 2011, 50, 4176–4179 CrossRef CAS PubMed.
- J. Yu, S. Choi and R. Dickson, Angew. Chem., Int. Ed., 2009, 48, 318–320 CrossRef CAS PubMed.
- A. Rotaru, S. Dutta, E. Jentzsch, K. Gothelf and A. Mokhir, Angew. Chem., Int. Ed., 2010, 49, 5665–5667 CrossRef CAS PubMed.
- X. Mao, S. Liu, C. Yang, F. Liu, K. Wang and G. Chen, Anal. Chim. Acta, 2016, 909, 101–108 CrossRef CAS PubMed.
- H. Li, J. Chang, T. Hou, L. Ge and F. Li, Talanta, 2016, 160, 475–480 CrossRef CAS PubMed.
- C. Yang, K. Shi, B. Dou, Y. Xiang, Y. Chai and R. Yuan, ACS Appl. Mater. Interfaces, 2015, 7, 1188–1193 CAS.
- W. Li, W. Li, Y. Hu, Y. Xia, Q. Shen, Z. Nie, Y. Huang and S. Yao, Biosens. Bioelectron., 2013, 47, 345–349 CrossRef CAS PubMed.
- R. Hu, Y. Liu, R. Kong, M. Donovan, X. Zhang, W. Tan, G. Shen and R. Yu, Biosens. Bioelectron., 2013, 42, 31–35 CrossRef CAS PubMed.
- G. Liu, Y. Shao, J. Peng, W. Dai, L. Liu, S. Xu, F. Wu and X. Wu, Nanotechnology, 2013, 24, 345502 CrossRef PubMed.
- X. Wang, B. Yin and B. Ye, RSC Adv., 2013, 3, 8633–8636 RSC.
- X. Liu, F. Wang, A. Niazov-Elkan, W. Guo and I. Willner, Nano Lett., 2013, 13, 309–314 CrossRef CAS PubMed.
- X. Zhu, H. Shi, Y. Shen, B. Zhang, J. Zhao and G. Li, Nano Res., 2015, 8, 2714–2720 CrossRef CAS.
- Z. Yuan, Y. Chen, H. Li and H. Chang, Chem. Commun., 2014, 50, 9800 RSC.
- S. Liang, Y. Kuang, F. Ma, S. Chen and Y. Long, Biosens. Bioelectron., 2016, 85, 758–763 CrossRef CAS PubMed.
- F. Chen, J. Tu, C. Liang, B. Yang, C. Chen, X. Chen and C. Cai, Microchim. Acta, 2016, 183, 1571–1577 CrossRef CAS.
- M. Shamsipur, F. Molaabasi, S. Hosseinkhani and F. Rahmati, Anal. Chem., 2016, 88, 2188–2197 CrossRef CAS PubMed.
- J. Wang, X. Wang, S. Wu, J. Song, Y. Zhao, Y. Ge and C. Meng, Anal. Chim. Acta, 2016, 906, 80–88 CrossRef CAS PubMed.
- J. Yin, X. He, X. Jia, K. Wang and F. Xu, Analyst, 2013, 138, 2350 RSC.
- H. Huang, H. Li, J. Feng and A. Wang, Sens. Actuators, B, 2016, 223, 550–556 CrossRef CAS.
- L. Deng, Z. Zhou, J. Li, T. Li and S. Dong, Chem. Commun., 2011, 47, 11065–11067 RSC.
- J. MacLean, K. Morishita and J. Liu, Biosens. Bioelectron., 2013, 48, 82–86 CrossRef CAS PubMed.
- J. Peng, J. Ling, X.-Q. Zhang, H. P. Bai, L. Zheng, Q. E. Cao and Z.-T. Ding, Spectrochim. Acta, Part A, 2015, 137, 1250–1257 CrossRef CAS PubMed.
- J. Obliosca, C. Liu, R. Batson, M. Babin, J. Werner and H. Yeh, Biosensors, 2013, 3, 185–200 CrossRef CAS PubMed.
- N. Enkin, F. Wang, E. Sharon, H. Albada and I. Willner, ACS Nano, 2014, 8, 11666–11673 CrossRef CAS PubMed.
- L. Feng, J. Liu, S. Zhang and X. Zhang, Anal. Methods, 2015, 7, 5689–5694 RSC.
- J. Wang, O. Rincón, R. Polsky and E. Dominguez, Electrochem. Commun., 2003, 5, 83–86 CrossRef CAS.
- H. Yeh, J. Sharma, J. Han, J. Martinez and J. Werner, Nano Lett., 2010, 10, 3106–3110 CrossRef CAS PubMed.
- N. Zhu, A. Zhang, P. He and Y. Fang, Analyst, 2003, 128, 260–264 RSC.
- X. Liu, F. Wang, R. Aizen, O. Yehezkeli and I. Willner, J. Am. Chem. Soc., 2013, 135, 11832–11839 CrossRef CAS PubMed.
- L. Zhang, J. Zhu, S. Guo, T. Li, J. Li and E. Wang, J. Am. Chem. Soc., 2013, 135, 2403–2406 CrossRef CAS PubMed.
- L. Feng, J. Liu, S. Zhang and X. Zhang, Anal. Methods, 2015, 7, 5689–5694 RSC.
- J. Ma, B. Yin, H. Le and B. Ye, ACS Appl. Mater. Interfaces, 2015, 7, 12856–12863 CAS.
- M. Dadmehr, M. Hosseini, S. Hosseinkhani, M. RezaGanjali and R. Sheikhnejad, Biosens. Bioelectron., 2015, 73, 108–113 CrossRef CAS PubMed.
- S. W. Yang and T. Vosch, Anal. Chem., 2011, 83, 6935–6939 CrossRef CAS PubMed.
- Y.-Q. Liu, M. Zhang, B.-C. Yin and B.-C. Ye, Anal. Chem., 2012, 84, 5165–5169 CrossRef CAS PubMed.
- C. Yang, K. Shi, B. Dou, Y. Xiang, Y. Chai and R. Yuan, ACS Appl. Mater. Interfaces, 2015, 7, 1188–1193 CAS.
- M. Zhang, Y.-Q. Liu, C.-Y. Yu, B.-C. Yin and B.-C. Ye, Analyst, 2013, 138, 4812–4817 RSC.
- W. Guo, J. Yuan, Q. Dong and E. Wang, J. Am. Chem. Soc., 2010, 132, 932–934 CrossRef CAS PubMed.
- X. Jia, J. Li, L. Han, J. Ren, X. Yang and E. Wang, ACS Nano, 2012, 6, 3311–3317 CrossRef CAS PubMed.
- X. Liu, R. Hu, Z. Gao and N. Shao, Langmuir, 2015, 31, 5859–5867 CrossRef CAS PubMed.
- Z. Huang, F. Pu, Y. Lin, J. Ren and X. Qu, Chem. Commun., 2011, 47, 3487–3489 RSC.
- X. Tian, X.-J. Kong, Z.-M. Zhu, T.-T. Chen and X. Chu, Talanta, 2015, 131, 116–120 CrossRef CAS PubMed.
- M. Zhang, S.-M. Guo, Y.-R. Li, P. Zuo and B.-C. Ye, Chem. Commun., 2012, 48, 5488–5490 RSC.
- J. Li, X. Zhong, H. Zhang, X. C. Le and J.-J. Zhu, Anal. Chem., 2012, 84, 5170–5174 CrossRef CAS PubMed.
- M. Duan, Y. Peng, L. Zhang, X. Wang, J. Ge, J. Jiang and R. Yu, Anal. Methods, 2013, 5, 2182–2187 RSC.
- G. Liu, D.-Q. Feng, X. Mu, W. Zheng, T. Chen, L. Qi and D. Li, J. Mater. Chem. B, 2013, 1, 2128–2131 RSC.
- H.-X. Han, X. Tian, X.-J. Kong, R.-Q. Yu and X. Chu, Anal. Methods, 2015, 7, 7989–7994 RSC.
- G.-Y. Lan, W.-Y. Chen and H. T. Chang, Analyst, 2011, 136, 3623–3628 RSC.
- Y. Zhou, T. Zhou, M. Zhang and G. Shi, Analyst, 2014, 139, 3122–3126 RSC.
- W. Li, W. Li, Y. Hu, Y. Xia, Q. Shen, Z. Nie, Y. Huang and S. Yao, Biosens. Bioelectron., 2013, 47, 345–349 CrossRef CAS PubMed.
- T. Ye, C. Li, C. Su, X. Ji and Z. He, RSC Adv., 2015, 5, 55336–55339 RSC.
- Y. Dou and X. Yang, Anal. Chim. Acta, 2013, 784, 53–58 CrossRef CAS PubMed.
- J. Chen, X. Zhang, S. Cai, D. Wu, M. Chen, S. Wang and J. Zhang, Biosens. Bioelectron., 2014, 57, 226–231 CrossRef CAS PubMed.
- Y. Chang, P. Zhang, Y. Yu, Y. Q. Du, W. Wang and C. Z. Huang, Anal. Methods, 2013, 5, 6200–6204 RSC.
- N. Enkin, E. Sharon, E. Golub and I. Willner, Nano Lett., 2014, 14, 4918–4922 CrossRef CAS PubMed.
- Y. Ma, S. Wang and L. Wang, TrAC, Trends Anal. Chem., 2015, 65, 13–21 CrossRef CAS.
- X. Sun, Y. Wang and Y. Lei, ChemInform, 2015, 44, 8019–8061 CAS.
- J. N. Tiwari, K. Nath, S. Kumar, R. N. Tiwari, K. C. Kemp, N. H. Le, D. H. Youn, J. S. Lee and K. S. Kim, Nat. Commun., 2013, 4, 1–7 Search PubMed.
- W. Liu, H. Lai, R. Huang, C. Zhao, Y. Wang, X. Weng and X. Zhou, Biosens. Bioelectron., 2015, 68, 736–740 CrossRef CAS PubMed.
- J.-L. Ma, B.-C. Yin and B.-C. Ye, RSC Adv., 2015, 5, 65437–66544 RSC.
- T. Li, L. Zhang, J. Ai, S. Dong and E. Wang, ACS Nano, 2011, 5(8), 6334–6338 CrossRef CAS PubMed.
- S. Choi, J. Yu, S. A. Patel, Y.-L. Tzeng and R. M. Dickson, Photochem. Photobiol. Sci., 2011, 10, 109–115 CAS.
- J. Yu, S. Choi and R. M. Dickson, Angew. Chem., Int. Ed. Engl., 2009, 48(2), 318–320 CrossRef CAS PubMed.
- Y. Antoku, J.-I. Hotta, H. Mizuno, R. M. Dickson, J. Hofkens and T. Vosch, Photochem. Photobiol. Sci., 2010, 9, 716–721 CAS.
- J. Yu, S. Choi, C. I. Richards, Y. Antoku and R. M. Dickson, Photochem. Photobiol., 2008, 84, 1435–1439 CrossRef CAS PubMed.
- J. Yin, X. He, K. Wang, F. Xu, J. Shangguan, D. He and H. Shi, Anal. Chem., 2013, 85, 12011–12019 CrossRef CAS PubMed.
- J. Li, X. Zhong, F. Cheng, J.-R. Zhang, L.-P. Jiang and J.-J. Zhu, Anal. Chem., 2012, 84, 4140–4146 CrossRef CAS PubMed.
- Z. Sun, Y. Wang, Y. Wei, R. Liu, H. Zhu, Y. Cui, Y. Zhao and X. Gao, Chem. Commun., 2011, 47, 11960–11962 RSC.
| This journal is © The Royal Society of Chemistry 2016 |