DOI:
10.1039/C6RA24079H
(Paper)
RSC Adv., 2016,
6, 111308-111317
Facile route to a conducting ternary polyaniline@TiO2/GN nanocomposite for environmentally benign applications: photocatalytic degradation of pollutants and biological activity
Received
28th September 2016
, Accepted 10th November 2016
First published on 11th November 2016
Abstract
A polyaniline@TiO2/graphene (Pani@TiO2/GN) nanocomposite was prepared by the in situ oxidative polymerization of aniline in the presence of TiO2 and GN nanoparticles. The resulting Pani@TiO2/GN nanocomposite was characterized by UV-visible diffuse absorbance/reflectance spectroscopy (DRS), photoluminescence spectroscopy (PL), transmission electron microscopy, scanning electron microscopy, X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS). The observance of peaks of Pani, TiO2 and GN in XRD and XPS as well as the observance of TiO2 nanoparticles well distributed inside the network of the Pani and GN nanosheets from morphological characterizations suggests the successful formation of Pani@TiO2/GN nanocomposites. DRS and PL analysis showed that Pani@TiO2/GN had higher visible light absorption and a lower recombination rate than Pani@TiO2. The visible light photocatalytic activity of the Pani@TiO2/GN nanocomposite was tested for methylene blue (MB) degradation. The results revealed high photocatalytic activity, which is partly due to the sensitizing effect of Pani and the low recombination rate due to the GN electron scavenging property. The rate of MB degradation on the Pani@TiO2/GN nanocomposite was strongly dependent on the solution pH, reaction time, catalyst dose, and the initial MB concentration. The high regeneration degradation efficiency of the Pani@TiO2/GN nanocomposite showed high stability and the effectiveness of the synthesized photocatalyst. In a continuation of environmental remediation studies, Pani@TiO2/GN revealed high antibacterial activity towards Escherichia coli and Enterobacter ludwigii, highlighting its potential as a photocatalyst with antibacterial properties for different industrial and medical purposes.
Introduction
Since Honda and Fujishima first reported the photo-oxidation of water on TiO2, the TiO2 nanostructures have attracted considerable research attention worldwide.1 Accordingly, TiO2 has been exploited widely for the photodegradation of organic pollutants in waste water owing to its exceptional optical properties, non-toxicity, low cost, and high stability towards photo and chemical corrosion.2 Nano particulate TiO2 is an efficient light-harvesting material with the potential for use in the photocatalytic removal of hazardous industrial byproducts and for photocatalytic water splitting. On the other hand, the solar energy to hydrogen production efficiency of TiO2 is limited substantially by its wide band gap energy, which means that it can utilize only ∼5% of solar light, and the high rate of electron and hole pair recombination. Thus, different strategies have been developed to tune the band gap response of TiO2 to the visible region. These include doping with dyes,3 metal ions,4 noble metals,5 non-metal or co-doping, deposition,6 composite formation with polymers, defect induction using ion beam irradiation, and coupling TiO2 with narrow band gap semiconductors.
Recently, nanocomposites of TiO2 with graphene (GN) have been reported as advanced photocatalytic materials because of their high performance in advanced areas.7–9 Vaclav Štengl et al.10 synthesized TiO2/GN nanocomposite by a one-pot process and revealed its high efficiency towards the mineralization of butane. Similar results were also reported by Zhou et al.,11 who observed the highly efficient degradation of methylene blue (MB) under sunlight irradiation. Zhang et al.12,13 showed that TiO2/GN photocatalyst under ambient conditions possessed higher activity and stability towards the gas-phase degradation of benzene in comparison to bare TiO2. However, in contrast to other reports they reported that TiO2/GN is similar to other TiO2 and carbon based composite (carbon nanotubes, fullerenes, and activated carbon) on enhancement of photocatalytic activity of TiO2. They reported that optimum doping of GN in TiO2 gives best results while a higher or very low doping may give TiO2/GN composites with poor photocatalytic properties. The high photo activity is because GN can work as a reservoir in electron capture/transport, which can boost the lifetime of photo excited electron–hole pairs from the semiconductor upon light irradiation, thereby increasing the process yield.
In our previous report, metal oxide composites with polyaniline (Pani) were employed successfully to achieve greater absorption of light in the UV and visible regions, which leads to the sensitization of metal oxides because Pani acts as a light harvester for metal oxide, such as TiO2.14 Hidalgo et al.15 reported that Pani acts as sensitizer of TiO2 by decreasing its band gap. Pani absorbs light and induces a π–π* transition and the excited electrons are transferred to the π* orbital and the excited electrons can be injected readily into the conduction band of TiO2 which lowers the band gap of TiO2. GN on the other hand due to its high conductivity and mobility can work as an electron scavenger which can decrease the recombination rate.16 Therefore, it is believed that a nanocomposite of GN, TiO2 with Pani, will exhibit enhanced photocatalytic activity due to the low band gap, low recombination rate and high absorption under UV and visible region, because of the synergism between the constituents.
Therefore, a nanocomposite of GN, TiO2 with Pani (Pani@TiO2/GN) was produced by the in situ oxidative polymerization of aniline in the presence of GN and TiO2 using dilute polymerization conditions in small volume of aniline. The prepared nanocomposite was used for the degradation of MB dye under visible light irradiation. The effects of the reaction time, solution pH, dye concentration, and photocatalyst dose were investigated. In a continuation of environmental remediation studies, Pani@TiO2/GN exhibited high antibacterial activity towards Escherichia coli and Enterobacter ludigii. Therefore, this nanocomposite can be used as a photocatalyst with antibacterial properties for a range of industrial and medical purposes.
Materials and method
Aniline, TiO2 (mean particle size ∼21 nm) from Sigma Aldrich and GN was purchased from Iljin Nano Tech, Seoul, Korea (thickness ∼8 nm and average length ∼500 nm). Potassium persulphate (PPS), HCl, and methanol were obtained from Duksan Pure Chemicals, Korea, and used as received. Methylene blue (MB) (C16H18N3SCl) was purchased from Techno pharma, Bahadurgarh, India. An aqueous solution of MB was prepared by dissolving 1.0 g of MB in 1.0 L water with a subsequent dilution to obtain the desired concentration of MB for the photocatalytic degradation studies. The water used in these experiments was de-ionized water from a PURE ROUP 30 water purification system.
Antimicrobial analysis
Pani@TiO2/GN and TiO2 were assessed for their antimicrobial activity by a modified well diffusion method and broth dilution method using nutrient agar and broth media. The materials were dissolved separately in diluted DMSO (water and DMSO 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio). Each compound was tested against Escherichia coli and Enterobacter ludwigii at different (0 to 50 mg) concentrations and incubated at 37 °C for 24 hours. The inocula of each microbial strain were prepared by suspending overnight grown cultures in normal saline (NaCl 0.85%). The turbidity of the inocula was adjusted as per the 0.5 McFarland standards.
1 ratio). Each compound was tested against Escherichia coli and Enterobacter ludwigii at different (0 to 50 mg) concentrations and incubated at 37 °C for 24 hours. The inocula of each microbial strain were prepared by suspending overnight grown cultures in normal saline (NaCl 0.85%). The turbidity of the inocula was adjusted as per the 0.5 McFarland standards.
The separately prepared and overnight sterility-checked nutrient agar plates were used in a well-diffusion assay. Each bacterial culture (100 μL) inocula were spread over the plate surface and after 15 minutes (time taken to absorb on the plate surface); 8 mm diameter wells were cut in the nutrient agar plates using sterile cork borer. The bottom of each well was sealed by adding 10 μL of molten agar (0.8%). For the antimicrobial assay, both materials with different concentrations were filled in the wells. Dilute DMSO was used as a negative control and the standard drug, amoxicillin, was used as the positive control. After overnight incubation in the incubator at 37 °C, all plates were examined and the diameter of the zone of inhibition was measured. In another method, a suspension of the materials (1 mg mL−1) was placed directly on the plate surface at different concentrations.
The antimicrobial activities of the both materials were investigated quantitatively through the different doses of materials supplemented in the growing media. To obtain an efficient dosage, after the incubation of treated liquid culture, 100 μL of the culture was removed and spread over the nutrient agar plates and the plates were incubated overnight at 37 °C. After incubation, the colony forming units (cfu) were recorded in the respective dilutions of all the treated samples. The above incubated broth cultures, including the blank samples devoid of materials were centrifuged at 3000 rpm for 1 min (sufficient for sedimentation of the materials) and the supernatant (presumably containing microbes) was separated to test the change in turbidity due to microbial growth by measuring the absorbance using a UV-Visible spectrophotometer. All the experiments were replicated 3 times.
Synthesis of Pani@TiO2/GN nanocomposites
The Pani@TiO2/GN nanocomposite was synthesized by the simple in situ oxidative polymerization of aniline in the presence of TiO2 under dilute polymerization conditions using a small amount of aniline monomer. In a typical process, a composite of TiO2 and GN (TiO2/GN) was prepared by dissolving 0.05 g GN and 1 g TiO2 in 50 mL of ethanol. The system was placed in an ultrasonic bath for 10 min and later on a hot plate with vigorous stirring until the solvent had evaporated. The prepared TiO2/GN nanocomposite was grinded with a mortar and pestle to obtain a homogenous powder, which was stored in a desiccator for further experiments. For further fabrication of TiO2/GN, the aniline monomer (0.5 g) was added to TiO2/GN and stirred vigorously for 2 h to allow the proper absorption of the aniline monomer to TiO2/GN. Subsequently, the solution of the oxidant (prepared in 1 M HCl) was added to affect the polymerization reaction. The reaction mixture transformed to greenish-black slurry, which was filtered after 2 hours, washed sequentially with distilled water, and excess ethanol to remove the dissolved impurities and Pani oligomers. The nanocomposite was de-doped with 1 M NH4OH and later doped by dispersing it in 1 M HCl for 24 hours. Subsequently, the prepared Pani@TiO2/GN nanocomposites were dried at 80 °C for 6 h in an air oven, converted to fine powders, and stored in a desiccator for further experiments. Pani@TiO2 was prepared in a similar manner but in the absence of GN.
Characterization
Methods
X-ray diffraction (XRD, PANalytical, X'Pert-PRO MPD) was performed using Cu Kα radiation (λ = 0.15405 nm). The microstructures were observed by field emission transmission electron microscopy (FE-TEM, Tecnai G2 F20 and scanning electron microscopy (SEM, HITACHI-S4800). The UV-visible diffuse absorbance was measured using a UV-VIS-NIR spectrophotometer (VARIAN, Cary 5000 U.S.A.). The photoluminescence (PL, Kimon, 1 K, Japan) spectra of the nanocomposites was recorded over the range, 200–800 nm, at an excitation wavelength of 325 nm and a power of 50 mW. X-ray photoelectron spectroscopy (XPS, ESCALAB 250) was performed using a monochromatized Al Kα X-ray source (hν = 1486.6 eV) with a 500 μm spot size. The photocatalytic degradation was performed in a Luzchem LZC 4V photoreactor using 112 W visible lamps.
Photocatalytic activity
The photocatalytic reactions were conducted in a 250 mL beaker containing 100 mL of a MB solution with continuous aeration and magnetic stirring. The effects of the solution pH and contact time were performed by mixing 0.05 g Pani@TiO2/GN with 100 mL dye solution of the initial concentration 10.5 mg L−1 at different pH and contact times ranging from 3 to 9 and 15 to 180 min, respectively. The effects of the initial MB concentration on the photocatalytic activity of Pani@TiO2/GN was studied by varying the initial dye concentration from 5 to 20 mg L−1 at a fixed solution pH 9 and a photocatalyst mass of 0.05 g. The effects of the photocatalyst dose on MB decomposition was also performed by adding different amounts of Pani@TiO2/GN, ranging from 0.01 to 0.07 g, in a 100 mL dye solution of 10.5 mg L−1 concentration at pH 9. The amount of MB remaining in the supernatant solution was determined at maximum wavelength 665 nm using a HACH LANGE DR-6000 UV-visible spectrometer.
Results and discussion
A simple in situ oxidative polymerization technique was used for the synthesis of the Pani@TiO2/GN nanocomposite. The synthesized Pani@TiO2/GN nanocomposite is expected to show enhanced photocatalytic activity because Pani shows high absorbance in the visible region, which when added to the properties of TiO2 and GN, may provide materials with hitherto unreported properties. Fig. 1 presents a schematic diagram of the synthesis of the Pani@TiO2/GN nanocomposite.
 |
| | Fig. 1 Schematic representation of the synthesis of Pani@TiO2/GN nanocomposite. | |
Morphological studies
The SEM images of the Pani@TiO2/GN revealed an agglomerated slightly distorted globular and tubular morphology along with the observance of some flaky structures (Fig. 2a and b). The morphology was distorted tubular globules due to the surface modification of TiO2 by GN flakes and the extension of interconnected globular tubules is due to the rapid polymerization conditions adopted, which makes the TiO2/GN grow into tubular structures covered with Pani. The inner nanostructure of the as-prepared Pani@TiO2/GN nanocomposite was examined by TEM (Fig. 2c). Clusters of TiO2 nanoparticles can be seen well distributed inside the network of the Pani and GN nanosheets. The porous structure of Pani on the TiO2 surface and elsewhere, as evident from the figure, is an important characteristic that allows specific interactions of the Pani@TiO2/GN nanocomposite with the dye molecules, making it an important feature for the photocatalytic performance. HR-TEM (Fig. 2d) revealed TiO2 crystals and GN with a high degree of structural uniformity. The SAED pattern also showed a distinct diffraction maximum and the sequential appearance of dark and bright fringes, which are characteristic of the polycrystalline structures of TiO2.17
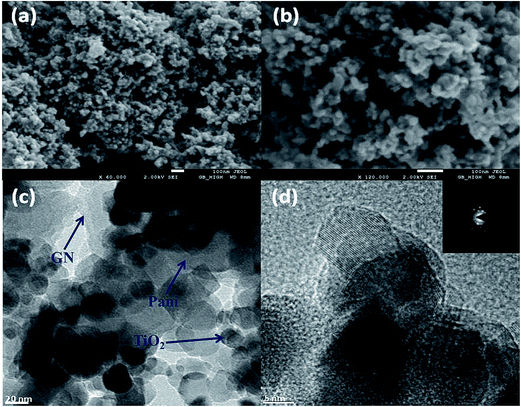 |
| | Fig. 2 SEM images at different magnifications (a, b), TEM (c) and HR-TEM (d) of Pani@TiO2/GN nanocomposite (the inset in (d) shows the SAED pattern). | |
Structural and compositional analysis. Fig. 3a presents the characteristic XRD peaks of the TiO2/GN and Pani@TiO2/GN nanocomposite. A sharp peak at 26.73 2θ can be attributed to TiO2 and is also very close to the typical diffraction peak of graphite 26.68 2θ, which might indicate the overlapping of peaks and the presence of both of both TiO2 and GN in the nanocomposite.18 All the peaks corresponding to the anatase and rutile phases of TiO2 are present in the XRD patterns.19 In the case of Pani@TiO2/GN, two major phenomena were observed, i.e., the characteristic peak of TiO2 decreased in intensity and the non-observance of a peak for Pani. The characteristic peaks of Pani at ∼15.1, 20.7 and 25.5° 2θ was not observed, which may be due to its amorphous nature and its small amount compared to TiO2 owing to the dilute polymerization conditions.20
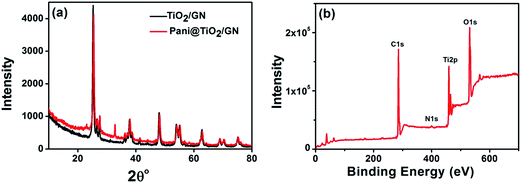 |
| | Fig. 3 XRD patterns of TiO2/GN and Pani@TiO2/GN (a) and XPS spectra of Pani@TiO2/GN nanocomposite (b). | |
XPS was conducted for composition and surface analysis of the Pani@TiO2/GN nanocomposite (Fig. 3b). The survey XPS spectrum revealed the existence of Ti, O, C, and N, corresponding to Pani, TiO2, and GN, suggesting the successful synthesis of Pani@TiO2/GN nanocomposite.
Optical study. Generally the PL technique is employed to probe the charge recombination and migration process occurred over the surface of the photocatalyst whereas the PL emission intensity generally displays the recombination process of the charge carriers. If the intensity of the PL emission is high it means the rapid charge recombination of the photogenerated charge carriers whereas weaker PL intensity means a lower recombination rate of electron–hole, which is favorable for enhancing the photocatalytic activity of the materials. Fig. 4a presents the PL emission spectra of the TiO2, TiO2/GN, and Pani@TiO2/GN nanocomposite. TiO2 exhibits the strongest emission intensity of the PL spectrum, confirming the fastest charge recombination rate, whereas in the case of TiO2/GN, the intensity is reduced significantly. Moreover, after coating with Pani, the intensity is reduced further. Lui et al.21 reported attributed the high photocatalytic performance in the case of GN-based composites to its ability to increase the conductivity by facilitating charge transfer, which suppresses recombination by accepting photo-generated electrons. Similarly, Pani also suppressed the electron recombination rate due to its conjugated π–π structures by facilitating charge transfer.14 This suggests that the photo-induced electrons and holes were trapped by the synergy between the GN energy level and Pani, which greatly suppresses the charge recombination rate of the photogenerated charges in Pani@TiO2/GN compared to TiO2/GN and TiO2.
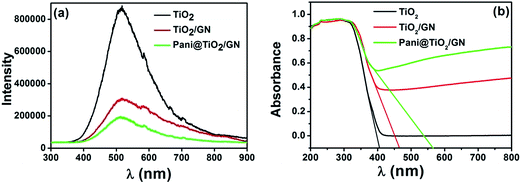 |
| | Fig. 4 (a) PL and (b) DRS spectra of TiO2, TiO2/GN and Pani@TiO2/GN nanocomposite. | |
Fig. 4b presents the UV-vis diffuse absorbance spectra of TiO2, TiO2/GN, and Pani@TiO2/GN nanocomposite. In the case of TiO2/GN, the red shift of absorbance was observed, which shifted further in Pani@TiO2/GN due to the change in the band gap with the GN loading and its further fabrication with Pani. A strong interaction between Pani and TiO2 via coordination between titanium and the nitrogen atom and the interaction of conjugated system with GN has been suggested, which suggests that these are possible sites of an interaction between TiO2 and Pani.22,23 The high degree of red shift in the case of Pani@TiO2/GN indicates the coordination of TiO2 with the nitrogen atom, and a possible π–π interaction between GN and Pani via the pi-conjugated system. The increase in absorption intensity after the incorporation of GN and Pani in Pani@TiO2/GN can be related to its better visible light activity than TiO2 and TiO2/GN. The absorbance spectra showed that TiO2 does not possess absorbance in the visible light region, but the absorbance increased for TiO2/GN and is enhanced further in the case of Pani@TiO2/GN, which revealed high visible light absorbance accounting for its high visible light photoactivity. The band gap calculated by the direct tangent drawing method24,25 was found to be 3.1, 2.6, and 2.1 eV for TiO2, TiO2/GN and Pani@TiO2/GN, respectively. The band gap reduction might be due to the wrapping of Pani on the TiO2/GN surface or due to the various types of interactions, as mentioned above. This also in accordance with previous reports.14,19
Photocatalytic experiments
Effect of solution pH and contact time. The solution pH and contact time have a major effect on the degradation of MB, as shown in Fig. 5. The photocatalytic degradation of MB increased with increasing solution pH and contact time. The higher degradation of MB at pH 9 can be explained based on the higher negative charge on the Pani@TiO2/GN in basic medium, which favors the interaction of cationic dye molecules. The concentration of MB decreased sharply from 10.5 to 4.6 mg L−1 after 30 min of visible light irradiation at pH 9. On the other hand, at a similar time, the concentration of dye remaining in solution was 10.43, 8.33, and 6.95 mg L−1 at pH 3, 5, and 7, respectively. According to previous research conducted by Li et al.26 Ayad and El-Nasr,27 and Chang et al.,28 GN, Pani, and TiO2 carry a negative charge under basic solution conditions, which interacts electrostatically with the positively charged MB, enhancing the photocatalytic degradation. Therefore, the decomposition of MB by Pani@TiO2/GN increases with increasing solution pH. Furthermore, in the presence of excess OH− (basic condition), the formation of hydroxyl radicals by the oxidation of Pani@TiO2/GN surface bound H2O and OH− by h+ may be possible [h+VB + OH− (H2O) → ˙OH + H+], which enhances the degradation of MB at pH 9 (˙OH + MB → colorless degradation products).29
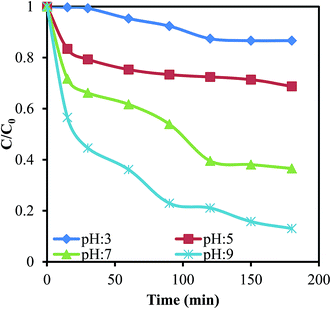 |
| | Fig. 5 Effect of the reaction time and solution pH on MB degradation onto Pani@TiO2/GN (Conc. – 10.5 mg L−1, V – 100 mL, catalyst dose – 0.05 g). | |
To predict the rate of MB photodegradation onto Pani@TiO2/GN, a pseudo-first-order kinetic model was applied using the Langmuir Hinshelwood equation: ln(C/C0) = kt, where C0 and C (mg L−1) are the initial and remaining MB concentrations at reaction time t (min). k (min−1) is the pseudo-first-order rate constant and is determined from the slope of −ln(C/C0) vs. t.30 The values of the rate constant k were calculated to be 0.001, 0.001, 0.004 and 0.008 min−1 at pH 3, 5, 7, and 9, respectively. The rate constant at pH 9 was highest compared to the other pH studied, confirming the fast and most favorable photocatalytic reaction at pH 9. Therefore, pH 9 was selected for further photocatalytic degradation studies.
Effect of Pani@TiO2/GN dosage. Fig. 6 shows the effects of the Pani@TiO2/GN dosage on the photocatalytic degradation of MB. The absorbance of MB deceases continuously with increasing catalyst dose, indicating a decrease in MB concentration and an increase in the degradation rate with increasing catalyst amount. The decomposition of MB increases from 49 to 91.6% with increasing Pani@TiO2/GN mass from 0.01 to 0.07 g (Fig. 6, inset). This behavior is likely due to the increase in the total number of active sites of Pani@TiO2/GN available for the photo reaction while the concentration of MB remained constant. A larger amount of catalyst generated a large number of e−/h+ pairs, which are responsible for the increase in MB degradation.31,32
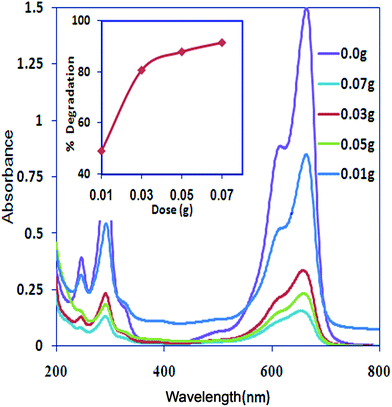 |
| | Fig. 6 Effect of the photocatalyst dose on MB degradation (inset: % degradation vs. catalyst dose plot) (Conc. – 10.5 mg L−1, pH – 9, V – 100 mL, time – 180 min). | |
Effect of the initial MB concentration. The photocatalytic reaction also has a great influence of the initial pollutant concentration. The effects of the initial MB concentration on photocatalytic degradation were investigated in the range, 5 to 20 mg L−1, and the results are presented in Fig. 7. The activity of Pani@TiO2/GN decreased gradually with increasing initial MB concentration. At low concentrations (5 mg L−1), almost 100% degradation of MB was observed but when the concentration was increased to 20 mg L−1, the degradation efficiency of Pani@TiO2/GN was 64.8%. This might be due to the high optical density in the solution at 5 mg L−1 and the large number of photons reaching the Pani@TiO2/GN surface.29,33 As the MB concentration was increased, the hindrance of light penetration in solution causes poor photocatalysis.31
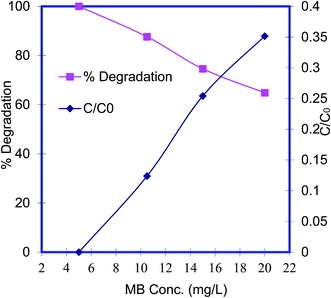 |
| | Fig. 7 Effect of the initial MB concentration on MB degradation (pH – 9, V – 100 mL, catalyst dose – 0.05 g, and time – 180 min). | |
Photocatalysis mechanism
The photocatalytic degradation of MB may take place through a series of reactions on the on the surface of the materials, such as (i) interaction of MB with Pani@TiO2/GN, (ii) intermediate products (photocatalysis), (iii) colorless degradation product, and (iv) saturation of Pani@TiO2/GN surface.34 In the present work, Pani@TiO2/GN is made of GN, TiO2, and Pani. All three components have their role in the degradation of MB on the surface of Pani@TiO2/GN.
Pani acts as a photosensitizer in Pani@TiO2/GN to sensitize the TiO2 surface. Under visible light irradiation, π–π transition occurred in Pani and electrons with highest occupied molecular orbital (HOMO) become excited and transfer to the lowest unoccupied molecular orbital (LUMO) of Pani. These electrons from the LUMO level are injected into the conduction band of TiO2, which react with O2 and generate O2˙− and HO2−˙ in the aqueous solution.19,35 Furthermore, the electrons from the conduction band of TiO2 and the LUMO of Pani may also be transferred to graphene. GN is a well-known electron acceptor material and used widely for reducing the band gap of TiO2 to make it active in visible region via the energetically favored hybridization of C 2p and O 2p atoms of GN and TiO2.36,37 These electrons on the GN are trapped by oxygen and water on the surface of Pani@TiO2/GN and produce the hydroxyl and superoxide radicals.38,39 Moreover, photogenerated hole in the HOMO level of Pani also generate hydroxyl radicals upon visible light excitation.40,41 Fig. 8 presents a brief mechanism of the transfer of the elections, generation of hole pairs, and the formation of oxidative radials.
| Pani + hν → Pani (e− + h+) |
| Pani (h+) + H2O → Pani + H+ + ˙OH |
| Pani (h+) + MB → Pani + degradation products |
| ˙OH + MB → degradation products |
| Pani (e−) + GN → Pani + GN (e−) |
| Pani (e−) + TiO2 → Pani + TiO2 (e−) |
| TiO2 (e−) + O2 → TiO2 + O2˙− |
| OH˙ + MB → degradation product |
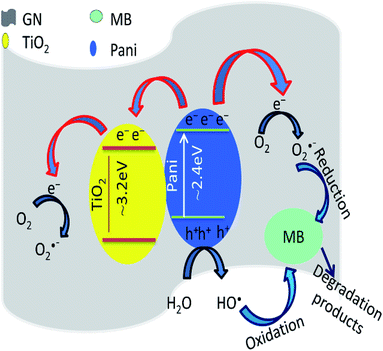 |
| | Fig. 8 Photodegradation mechanism of MB on Pani@TiO2/GN. | |
Comparison of photocatalytic activity
The photocatalytic activity of the pure TiO2, GN/TiO2 and Pani@TiO2 was investigated by taking the 100 mL of 10 mg L−1 MB solution in a beaker containing 0.05 g catalyst at pH 9 and reaction time was set to 180 min under continuous aeration and stirring. The results showed that pure TiO2 has very poor photocatalytic activity due to its inactivity in the visible light while GN/TiO2 and Pani@TiO2 showed ∼65 and ∼53% degradation of MB under visible light irradiation. Based on these results it can be concluded that Pani@GN/TiO2 is batter catalyst than the GN/TiO2 and Pani@TiO2 under visible light irradiation. Moreover, to find the effectiveness of Pani@GN/TiO2, photocatalytic activity of this composite was compared with the previously reported works as shown Table 1. It should be mentioned that the photocatalytic efficiency of the catalysts highly depends on the reaction conditions such as solution pH, reaction time, solution volume, catalyst mass dye concentration, type and intensity of radiation source etc. The results in Table 1, demonstrate that photocatalytic activity of the materials varies as the reaction conditions changes. However, it can be seen that on comparison to the previous studies, Pani@GN/TiO2 shows good catalytic activity as we have used the mild reaction condition such as comparatively low energy radiation source and catalyst mass.
Table 1 Comparison of the photocatalytic activity of various catalyst used for the degradation of MBa
| Catalyst |
Conditions |
Radiation |
% deg. |
Ref. |
| v – volume (mL), c – concentration. (mg L−1), m – catalyst mass (mg), t – time (min), V – Visible light, UV – ultraviolet light. |
| FeOOH-LDO |
v – 50, c – 3.0, m – 175, t – 210 |
500 W-V |
95 |
42 |
| ZnFeAl-LDO |
v – 50, c – 3.0, m – 175, t – 210 |
500 W-V |
60 |
42 |
| ZnAl-LDO |
v – 50, c – 3.0, m – 175, t – 210 |
500 W-V |
23 |
42 |
| Ag/TiO2 |
v – 100, c – 5.0, m – 100, t – 180, pH – 9 |
98 W-UV |
90 |
43 |
| ZnS–CdS |
v – 600, c – 10.0, m – 100, t – 360 |
500 W-V |
73 |
44 |
| ZIF-8 |
v – 50, c – 10.0, m – 25, t – 120, pH – 12 |
500 W-UV |
∼100 |
45 |
| Ag2O/TiO2@polypyrrole |
v – 100, c – 20.0, m – 50, t – 240, pH – 9 |
104 W-V |
100 |
46 |
| rGO–Fe3O4–TiO2 |
v – 100, c – 1, m – 50, t – 5 |
125 W-V |
91 |
47 |
| rGO–Fe3O4–TiO2 |
v – 100, c – 1, m – 50, t – 5 |
125 W-UV |
100 |
47 |
| GO–TiO2 |
v – 50, c – 10, m – 5, t – 5 |
4800 μW cm−2-UV |
91.2 |
48 |
| GN/TiO2 |
v – 100, c – 10.5, m – 50, t – 180, pH – 9 |
112 W-V |
65 |
This study |
| Pani@TiO2 |
v – 100, c – 10.5, m – 50, t – 180, pH – 9 |
112 W-V |
53 |
This study |
| Pani@GN/TiO2 |
v – 100, c – 10.5, m – 50, t – 180, pH – 9 |
112 W-V |
87 |
This study |
Regeneration of spent photocatalyst
The regeneration and reusability of Pani@TiO2/GN was also tested for the making the process effective and economical. For regeneration, the spent Pani@TiO2/GN catalyst was treated with 100 mL of 1.0 M HCl for 3 h and then centrifuged and washed thoroughly with water prior to using for each cycle. The reusability of Pani@TiO2/GN was tested three times and the results showed approximately 84.41, 80.11, and 76.28% of MB degradation after the first, second and third cycle, respectively. These results confirm that the Pani@TiO2/GN remain stable and effective, even after three times utilization.
Anti-bacterial studies
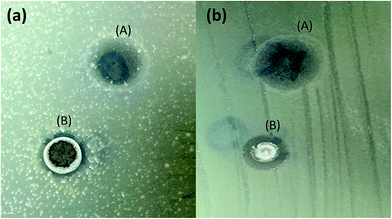
The present study analyzed the antibacterial effects of Pani@TiO2/GN and TiO2 against two pathogenic bacteria, Escherichia coli and Enterobacter ludwigii. Both bacterial organisms are most common in enteric infections and water pollution and are resistant to most broad spectrum new generation antibiotics. In addition, nanomaterials damage the cellular function by denaturation the cell enzymes, functional molecules, and protein factors. TiO2 and Pani@TiO2/GN were applied for antimicrobial activity against two enteric pathogenic bacteria, E. coli and E. ludwigii. Both TiO2 and Pani@TiO2/GN were applied to growth control in both liquid and solid medium. On the solid agar nutrient media, both nanomaterials showed antimicrobial activity, but Pani@TiO2/GN showed much higher antimicrobial activity compared to TiO2 (Fig. 9). The zone of inhibition was 17 and 12 mm for Pani@TiO2/GN and TiO2, respectively against both bacteria, E. coli and E. ludwigii, respectively, in a well diffusion assay. Furthermore, both Pani@TiO2/GN and TiO2 (1 mg μL−1) were applied directly to bacterial cultured plate and when observed after an overnight period of time, a successfully controlled bacterial growth, as depicted in Fig. 10, was observed. The antimicrobial activity of both Pani@TiO2/GN and TiO2 was also analyzed in the liquid media at 5 and 10 mg mL−1 concentration by measuring the optical density using a spectrophotometer at 600 nm. After overnight incubation, a sharp decline in bacteria growth was observed compared to the control bacteria (Fig. 11). During the investigation, after 28 h incubation of the treated and untreated culture separately, the optical density of the untreated culture was >1 while the optical density of the treated culture decreased significantly to <0.01. The reduced optical density clearly indicates the decline in turbidity due to the loss of bacterial growth. The findings are in accordance with previous studies on Pani-based materials, such as Pani@TiO2/CTAB and Pani/PVA/Ag.49,50
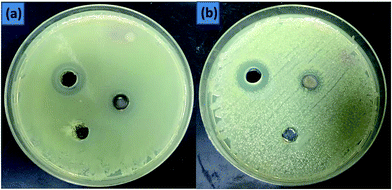 |
| | Fig. 9 Antibacterial activity of TiO2 and Pani@TiO2/GN (10 mg per well) against E. coli (a) and E. ludwigii (b) Pani@TiO2/GN showed excellent zone of inhibition ∼17 nm and TiO2 revealed 12 mm against both bacteria. | |
 |
| | Fig. 10 Antimicrobial activity of (1 mg) Pani@TiO2/GN (A) and TiO2 (B) on growing bacteria on the surface plate (a) E. coli and (b) E. ludwigii. | |
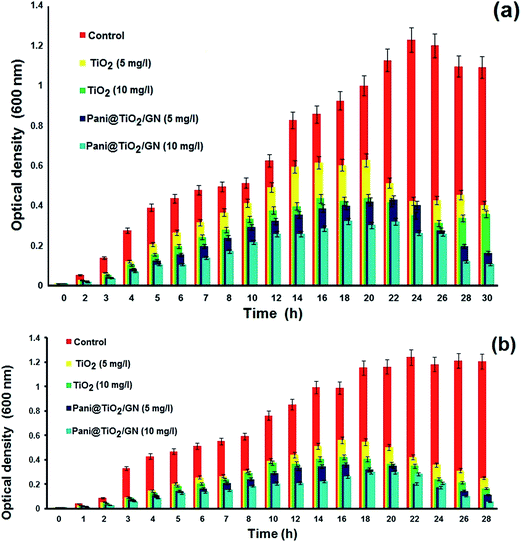 |
| | Fig. 11 Antimicrobial activity of TiO2 and Pani@TiO2/GN in liquid media at different concentrations from 0 to 10 mg mL−1 for (a) E. coli and (b) E. ludwigii. | |
Conclusion
Pani@TiO2/GN nanocomposite with enhanced environmentally benign applications, i.e., high photocatalytic activity and antibacterial properties, were fabricated using an in situ chemical oxidative polymerization method. Morphological characterization of the resulting nanocomposite revealed fibrous globular structures with well dispersed TiO2 and GN in the Pani matrix. The PL and DRS studies showed that the Pani@TiO2/GN nanocomposite possessed a much lower recombination rate of charge carriers and high visible light activity. All the components, i.e., GN, TiO2, and Pani played a crucial role in the degradation of degradation MB on the surface of the Pani@TiO2/GN catalyst. The results showed that the maximum degradation of MB on Pani@TiO2/GN was observed at pH 9 within 180 min. Kinetic studies showed that the degradation of MB followed a pseudo-first order kinetic model. Moreover, the rate of MB degradation decreases with increasing initial dye concentration and increases with increasing catalyst dose. The reusability and regeneration studies showed that Pani@TiO2/GN is an efficient and stable catalyst that can be used for environmental remediation applications. Pani@TiO2/GN also showed enhanced antibacterial activity towards Escherichia coli and Enterobacter ludwigii and has a high potential for applications in multiple fields of industrial use.
Acknowledgements
This study was supported by Priority Research Centers Program (Grant No. 2014R1A6A1031189), and by Basic Science Research Program (Grant No. 2015R1D1A3A03018029) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education. The authors also gratefully acknowledge King Abdulaziz University (KAU, Saudi Arabia) for funding this work.
References
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS PubMed
 .
. - J. Tian, Z. Zhao, A. Kumar, R. I. Boughton and H. Liu, Chem. Soc. Rev., 2014, 43, 6920 RSC
 .
. - K. Sayama, S. Tsukagoshi, K. Hara, Y. Ohga, A. Shinpou, Y. Abe, S. Suga and H. Arakawa, J. Phys. Chem. B, 2002, 106, 1363 CrossRef CAS
 .
. - S. K. Giri and N. N. Das, Powder Technol., 2013, 239, 193–198 CrossRef CAS
 .
. - F. Xiao, J. Mater. Chem., 2012, 22, 7819 RSC
 .
. - N. Zhang, C. Han, Y.-J. Xu, J. J. Foley IV, D. Zhang, J. Codrington, S. K. Gray and Y. Sun, Nat. Photonics, 2016, 10, 473 CrossRef CAS
 .
. - G. Williams, B. Seger and P. V. Kamat, ACS Nano, 2008, 2, 1487 CrossRef CAS PubMed
 .
. - X. Xie, K. Kretschmer and G. Wang, Nanoscale, 2015, 7, 13278 RSC
 .
. - C. Han, N. Zhang and Y.-J. Xu, Nano Today, 2016, 11, 351 CrossRef CAS
 .
. - V. Štengl, D. Popelková and P. Vláčil, J. Phys. Chem. C, 2011, 115, 25209 Search PubMed
 .
. - K. Zhou, Y. Zhu, X. Yang, X. Jiang and C. Li, New J. Chem., 2011, 35, 353 RSC
 .
. - Y. Zhang, Z.-R. Tang, X. Fu and Y.-J. Xu, ACS Nano, 2010, 4, 7303 CrossRef CAS PubMed
 .
. - N. Zhang and Y.-J. Xu, CrystEngComm, 2016, 18, 24 RSC
 .
. - M. O. Ansari, M. M. Khan, S. A. Ansari, J. Lee and M. H. Cho, RSC Adv., 2014, 4, 23713 RSC
 .
. - D. Hidalgo, S. Bocchini, M. Fontana, G. Saracco and S. Hernández, RSC Adv., 2015, 5, 49429 RSC
 .
. - N. Zhang, M.-Q. Yang, S. Liu, Y. Sun and Y.-J. Xu, Chem. Rev., 2015, 115, 10307 CrossRef CAS PubMed
 .
. - M. O. Ansari, M. M. Khan, S. A. Ansari and M. H. Cho, New J. Chem., 2015, 39, 8381 RSC
 .
. - M. O. Ansari, S. K. Yadav, J. W. Cho and F. Mohammad, Composites, Part B, 2013, 47, 155 CrossRef CAS
 .
. - M. O. Ansari, M. M. Khan, S. A. Ansari, K. Raju, J. Lee and M. H. Cho, ACS Appl. Mater. Interfaces, 2014, 6, 8124 CAS
 .
. - M. O. Ansari, M. M. Khan, S. A. Ansari and M. H. Cho, Electron. Mater. Lett., 2015, 11, 559 CrossRef CAS
 .
. - G. Lui, J. Y. Liao, A. Duan, Z. Zhang, M. Fowler and A. Yu, J. Mater. Chem. A, 2013, 1, 12255 CAS
 .
. - M. O. Ansari and F. Mohammad, Sens. Actuators, B, 2011, 157, 122 CrossRef CAS
 .
. - M. O. Ansari, M. M. Khan, S. A. Ansari, I. Amal, J. Lee and M. H. Cho, Mater. Lett., 2014, 114, 159 CrossRef CAS
 .
. - M. M. Khan, S. A. Ansari, D. Pradhan, M. O. Ansari, D. H. Han, J. Lee and M. H. Cho, J. Mater. Chem. A, 2014, 2, 637 CAS
 .
. - J. S. Lee, K. H. You and C. B. Park, Adv. Mater., 2012, 24, 1084 CrossRef CAS PubMed
 .
. - T. Liu, Y. Li, Q. Du, J. Sun, Y. Jiao, G. Yang, Z. Wang, Y. Xia, W. Zhang, K. Wang, H. Zhu and D. Wu, Colloids Surf., B, 2012, 90, 197 CrossRef CAS PubMed
 .
. - M. M. Ayad and A. A. El-Nasr, J. Phys. Chem. C, 2010, 114, 14377 CAS
 .
. - H. Chang, C. Su, C. H. Lo, L. C. Chen, T. T. Tsung and C. S. Jwo, Mater. Trans., 2004, 45, 3334 CrossRef CAS
 .
. - L. B. Reutergådh and M. Iangphasuk, Chemosphere, 1997, 35, 585 CrossRef
 .
. - W. Yao, B. Zhang, C. Huang, C. Ma, X. Song and Q. Xu, J. Mater. Chem., 2012, 22, 4050 RSC
 .
. - S. Mozia, A. W. Morawski, M. Toyoda and T. Tsumura, Chem. Eng. J., 2009, 150, 152 CrossRef CAS
 .
. - H. Huang, D. Y. C. Leung, P. C. W. Kwong, J. Xiong and L. Zhang, Catal. Today, 2013, 201, 189 CrossRef CAS
 .
. - R. W. Matthews, Water Res., 1991, 25, 1169 CrossRef CAS
 .
. - M. Ghaffari, P. Y. Tan, M. E. Oruc, O. K. Tan, M. S. Tse and M. Shannon, Catal. Today, 2011, 161, 70 CrossRef CAS
 .
. - Q. Wang, J. Hui, J. Li, Y. Cai, S. Yin, F. Wang and B. Su, Appl. Surf. Sci., 2013, 283, 577 CrossRef CAS
 .
. - R. K. Upadhyay, N. Soin and S. S. Roy, RSC Adv., 2014, 4, 3823 RSC
 .
. - N. Zhang, Y. Zhang and Y. J. Xu, Nanoscale, 2012, 4, 5792 RSC
 .
. - W. Gao, M. Wang, C. Ran, X. Yao, H. Yang, J. Liu, D. He and J. Bai, Nanoscale, 2014, 6, 5498 RSC
 .
. - E. Subramanian, S. Subbulakshmi and C. Murugan, Mater. Res. Bull., 2014, 51, 128 CrossRef CAS
 .
. - F. Wang, S. Min, Y. Han and L. Feng, Superlattices Microstruct., 2010, 48, 170 CrossRef CAS
 .
. - B. Haspulat, A. Gülce and H. Gülce, J. Hazard. Mater., 2013, 260, 518 CrossRef CAS PubMed
 .
. - S. Xia, L. Zhang, G. Pan, P. Qian and Z. Ni, Phys. Chem. Chem. Phys., 2015, 17, 5345 RSC
 .
. - R. Kumar, J. Rashid and M. A. Barakat, Colloids Interface Sci. Commun., 2015, 5, 1 CrossRef CAS
 .
. - N. Soltani, E. Saion, M. Z. Hussein, M. Erfani, A. Abedini, G. Bahmanrokh, M. Navasery and P. Vaziri, Int. J. Mol. Sci., 2012, 13, 12242 CrossRef CAS PubMed
 .
. - H.-P. Jing, C.-C. Wang, Y.-W. Zhang, P. Wang and R. Lia, RSC Adv., 2014, 4, 54454 RSC
 .
. - R. Kumar, J. Colloid Interface Sci., 2016, 477, 83 CrossRef CAS PubMed
 .
. - P. Benjwal, M. Kumar, P. Chamolia and K. K. Kar, RSC Adv., 2015, 5, 73249 RSC
 .
. - X. Luan, M. T. G. Wing and Y. Wang, Mater. Sci. Semicond. Process., 2015, 30, 592 CrossRef CAS
 .
. - E. A. O. Farias, N. A. Dionisio, P. V. Quelemes, S. H. Leal, J. M. E. Matos, E. C. S. Filho, I. H. Bechtold, J. R. S. A. Leite and C. Eiras, Mater. Sci. Eng., C, 2014, 35, 449 CrossRef CAS PubMed
 .
. - M. G. Moghaddam and H. Eslahi, Arabian J. Chem., 2014, 7, 846 CrossRef
 .
.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio). Each compound was tested against Escherichia coli and Enterobacter ludwigii at different (0 to 50 mg) concentrations and incubated at 37 °C for 24 hours. The inocula of each microbial strain were prepared by suspending overnight grown cultures in normal saline (NaCl 0.85%). The turbidity of the inocula was adjusted as per the 0.5 McFarland standards.
1 ratio). Each compound was tested against Escherichia coli and Enterobacter ludwigii at different (0 to 50 mg) concentrations and incubated at 37 °C for 24 hours. The inocula of each microbial strain were prepared by suspending overnight grown cultures in normal saline (NaCl 0.85%). The turbidity of the inocula was adjusted as per the 0.5 McFarland standards.





.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.






