Synthesis and solvent-free polymerisation of vinyl terephthalate for application as an anode material in organic batteries†
Djawed Nauroozi *a,
Marijana Pejicb,
Pierre-Olivier Schwartza,
Mario Wachtlerb and
Peter Bäuerle*a
*a,
Marijana Pejicb,
Pierre-Olivier Schwartza,
Mario Wachtlerb and
Peter Bäuerle*a
aInstitute of Organic Chemistry II and Advanced Materials, Ulm University, Albert-Einstein-Allee 11, D-89081 Ulm, Germany. E-mail: djawed.nauroozi@uni-ulm.de; peter.baeuerle@uni-ulm.de
bZSW – Zentrum für Sonnenenergie- und Wasserstoff-Forschung, Helmholtzstr. 8, D-89081 Ulm, Baden-Württemberg, Germany
First published on 17th November 2016
Abstract
The synthesis and polymerisation of dimethyl 2-vinylterephthalate M1 for possible applications as an anode material in organic secondary batteries are reported. M1 exhibits a vinyl group as a polymerisable unit while the carboxylate moieties serve as cation (Li+, Na+) coordinating sites. The gram-scale synthesis of M1 is described via three different routes in order to evaluate the route with the highest overall yield. Furthermore, different conditions for free radical polymerisation are investigated for obtaining polymer P1 with high molecular weights in order to study the impact of immobilising the carboxylate redox-active centres in a polymer on the charge/discharge cycling stability when used in an organic battery. In order to synthesis suitable materials for battery investigations, P1 was post-functionalised to the corresponding lithium salt P1-Li, which was further electrochemically investigated. Cyclic voltammetry measurements showed for P1-Li redox activity in the range of 0.5–1.2 V vs. Li+/Li which assigns it as a candidate for the anode. Under the present experimental conditions, the galvanostatic measurements of P1-Li exhibited a specific capacity of 64 mA h g−1. It is further demonstrated that P1-Li shows an improved cycling stability of 83% discharge capacity remaining after 100 cycles compared to the parent monomer (44%).
Introduction
The concept of using organic molecules as candidates for energy storage applications has recently attracted increased attention both for environmental and economic reasons.1–4 Compared to conventional inorganic electrode materials they offer a potentially low-cost production due to the low temperature procedures, their composition of naturally abundant chemical elements (C, H, N, O, S) that do not involve expensive metals, and their recyclability. Thus, organic materials represent a promising alternative to their inorganic counterparts.The access to an inexhaustible pool of organic molecules further allows the design of electrode materials suitable for a wide range of redox potentials. Several classes of organic compounds such as conjugated polymers, stable organic radicals, organodisulfides, and thioethers have already been studied.5–14 Recently, particular interest is focussed on organic carbonyls as they present a common functional group and are able to stabilise charges via delocalisation.3,15 Among carbonyl compounds, conjugated dicarboxylates such as terephthalates have been shown to be suitable candidates for anodes due to their electrochemical reactivity at low potentials.16 However, most of the organic electrode materials with low molecular weight suffer capacity fading after a while due to their solubility in the electrolyte. An intuitive approach to overcome this disadvantage is to use the corresponding polymers.17,18 Several polymers that show stable performance over some hundred cycles have already been investigated as electrode materials.19–21 However, there are just a few examples of polymers that can be used as anodes.22–24
Poly(terephthalates) such as poly(ethylene terephthalate), known as PET, are among the most common polymers, which are used today. They are produced via condensation reaction of a carboxylic acid and an alcohol. In order to use poly(terephthalate) as an electrode active material though, the carboxylate moiety needs to be accessible for the insertion/de-insertion of metal cations (Li+ or Na+) during the charge/discharge process in a battery. We therefore sought the synthesis of a styrene derivative, 2-vinyl-terephthalate M1 (Scheme 1), that allows the polymerisation of the terephthalate via the vinyl group while the carboxylate moiety remains accessible for the electrochemical processes.
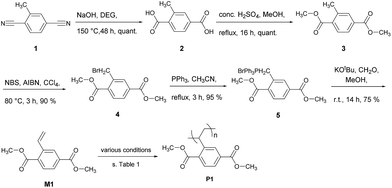 | ||
| Scheme 1 Synthesis of vinyl terephthalate monomer M1. For the polymerisation conditions towards P1, see text and Table 1. | ||
In this work, we report synthesis of terephthalate-based monomer M1 as well as the investigation of different conditions for free radical polymerisation in order to obtain the corresponding polymers in high molecular weights. The suitability and possible application of the synthesised polymer as anode material are further investigated by electrochemical methods such as cyclic voltammetry and galvanostatic cycling.
Experimental
Methods and instruments
NMR spectra were recorded on a Bruker AMX 500 (1H NMR: 500 MHz, 13C NMR: 125 MHz) or an Avance 400 spectrometer (1H NMR: 400 MHz, 13C NMR: 100 MHz), normally at 25 °C. Chemical shift values (δ) are expressed in parts per million using solvent signals (1H NMR, δH = 7.26 and 13C NMR, δC = 77.0 for CDCl3; 1H NMR, δH = 2.50 and 13C NMR, δC = 39.52 for DMSO-d6) as internal standards. Melting points were determined using a Büchi M-565. Elemental analyses were performed on an Elementar Vario EL (Ulm University). Cyclic voltammetry experiments were performed with an Autolab Potentiostat Galvanostat in a three-electrode single-compartment cell with a platinum working electrode, a platinum wire counter electrode, and an Ag/AgCl reference electrode. Ferrocene (Fc) has been added as internal reference. The potentials have furthermore been recalculated to the Li+/Li reference electrode using 3.25 V for the oxidation potential of ferrocene vs. Li+/Li. FT-IR spectra were measured with a Perkin Elmer Spectrum 2000. MS/CI experiments were performed using a Finnigan MAT, SSQ-7000 spectrometer.The battery-related electrochemical investigations were carried out in three-electrode Swagelok-type cells using lithium metal (Sigma Aldrich) as the counter and reference electrodes, 3 glass-fibre sheets (Whatman, GF/A) as separators, and 1 M LiPF6 in a mixture of ethylene carbonate and dimethyl carbonate (EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC) 1
DMC) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by wt (UBE Industries) as the electrolyte. The electrodes were prepared via a solvent-free preparation method using poly-tetrafluoroethylene (PTFE, Dyneon) as the binder. The active material (56.6 wt%) and carbon black (SuperP-Li, Timcal; 33 wt%) were homogenised by ball-milling (400 rpm, 10 min) before PTFE powder (10.4 wt%) was added in a mortar to form a smooth paste. After calendering, composite electrode discs with diameters of 12 mm and a typical thickness of 170–190 μm were cut out of the film, pressed onto aluminium expanded metal mesh (3 t; 1 min), then dried overnight under dynamic vacuum at 80 °C, and finally stored under dry Ar. The cells were assembled in an Ar-filled glove box. Cyclic voltammetry was performed with a VMP3 electrochemical workstation from Biologic. The experiments were run with a scan rate of 0.1 mV s−1 and a potential range of 0.5–2.0 V vs. Li+/Li. Galvanostatic cycling tests were performed with a CTS battery cycler from Basytec. The cells were charged and discharged with a constant current of 0.1C for 100 cycles, within 0.5–2.0 V vs. Li+/Li. The C-rates were calculated based on the theoretical capacity of the corresponding active material (theoretical capacity of P1-Li = 263 mA h g−1, 1C rate = 263 mA g−1, C/3 rate = 87.7 mA g−1). All electrochemical measurements were carried out at room temperature and repeated in order to guarantee reproducibility. The specific currents and capacities are referred to the mass of active material.
1 by wt (UBE Industries) as the electrolyte. The electrodes were prepared via a solvent-free preparation method using poly-tetrafluoroethylene (PTFE, Dyneon) as the binder. The active material (56.6 wt%) and carbon black (SuperP-Li, Timcal; 33 wt%) were homogenised by ball-milling (400 rpm, 10 min) before PTFE powder (10.4 wt%) was added in a mortar to form a smooth paste. After calendering, composite electrode discs with diameters of 12 mm and a typical thickness of 170–190 μm were cut out of the film, pressed onto aluminium expanded metal mesh (3 t; 1 min), then dried overnight under dynamic vacuum at 80 °C, and finally stored under dry Ar. The cells were assembled in an Ar-filled glove box. Cyclic voltammetry was performed with a VMP3 electrochemical workstation from Biologic. The experiments were run with a scan rate of 0.1 mV s−1 and a potential range of 0.5–2.0 V vs. Li+/Li. Galvanostatic cycling tests were performed with a CTS battery cycler from Basytec. The cells were charged and discharged with a constant current of 0.1C for 100 cycles, within 0.5–2.0 V vs. Li+/Li. The C-rates were calculated based on the theoretical capacity of the corresponding active material (theoretical capacity of P1-Li = 263 mA h g−1, 1C rate = 263 mA g−1, C/3 rate = 87.7 mA g−1). All electrochemical measurements were carried out at room temperature and repeated in order to guarantee reproducibility. The specific currents and capacities are referred to the mass of active material.
Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 220 mg (4 eq.) NaOH was added to the solution. The mixture was heated to reflux for 14 h. A precipitate was formed which was collected and washed excessively with MeOH. The product was obtained after drying under vacuum as a colourless powder in 80% yield (210 mg). FT-IR (KBr, 25 °C, cm−1): γ = 2200–3500, 1702, 1568, 1495, 1419, 1233, 1125, 1054, 916, 880, 853, 757.
1) and 220 mg (4 eq.) NaOH was added to the solution. The mixture was heated to reflux for 14 h. A precipitate was formed which was collected and washed excessively with MeOH. The product was obtained after drying under vacuum as a colourless powder in 80% yield (210 mg). FT-IR (KBr, 25 °C, cm−1): γ = 2200–3500, 1702, 1568, 1495, 1419, 1233, 1125, 1054, 916, 880, 853, 757.Results and discussion
Synthesis of the monomer
The synthesis of monomer M1 is shown in Scheme 1. Although molecules 1–5 as well as M1 are literature-known (s. ESI†), for some of them a synthesis procedure and/or proper characterisation are missing, which is described here. In the first step, the commercially available 2-methylterephthalonitrile 1 was treated with NaOH in presence of diethylene glycol (DEG) to obtain 2-methylterephthalic acid 2 in quantitative yield. Subsequent esterification under acidic conditions gave methyl ester 3 quantitatively. Bromination of the methyl group using N-bromosuccinimide (NBS) and subsequent transformation to the corresponding phosphonium salt provided 5 that serves as a precursor for the following Wittig reaction.25 Unfortunately, a convenient synthesis of monomer M1 with commercially available formaldehyde is not possible due to the presence of water in the methanol solutions of formaldehyde. To circumvent this obstacle, formaldehyde was generated in situ by decomposition of the para-formaldehyde polymer into the corresponding monomers at 200 °C. Thus, the formaldehyde was obtained as a gas, which could be easily directed to the reaction flask, where the phosphonium salt was already treated with potassium tert-butylate. This reaction gave the desired 2-vinylterephthalate M1 after 14 h reaction time at room temperature. Purification via flash chromatography and recrystallisation from ethyl acetate gave the monomer M1 in 75% yield. Although the overall yield of this multi-step synthesis is satisfying (65% over 5 steps), other routes have also been investigated in order to reduce the number of synthetic steps and to gain better yields (details in ESI†). However, the overall yields of the two other routes were less than the one shown here.Polymerisation experiments
The solubility of organic molecules in electrolytes is among the most serious obstacles for their use in batteries. On the one hand, the electrode capacity decreases as it dissolves, on the other hand, the dissolved redox-active molecules can act as redox shuttles resulting in a self-discharge of the cell. An intuitive approach to circumvent this problem is the transformation of the electrode-active material into a polymer. With the use of M1 we intended to prepare stable polymers P1 that show low solubility in the electrolyte mixtures of a battery (Scheme 1, last step). Hence, different conditions for a free radical polymerisation reaction of monomer M1 with 2,2′-azobis(2-methylpropionitrile) (AIBN) as radical starter have been investigated. All polymerisation conditions as well as the corresponding characterisation data for the obtained polymers are summarised in Table 1. The GPC chromatograms are shown in Fig. 1. First attempts to polymerise M1 in THF as solvent at 60 °C gave polymers with molecular weights of around Mn = 18 kDa corresponding to approximately 90 repeating units (Fig. 1, black curve). In order to obtain polymers with higher molecular weights, we tried to improve the polymerisation process by changing the solvent from THF to dioxane or N-methylpyrrolidone (NMP), which allow performing the reaction at higher temperatures, 100 °C and 150 °C, respectively. However, both solvents gave polymers with molecular weights of approximately 15 kDa (Fig. 1, orange and turquoise curves). The same trend was observed when different radical starters such as dibenzoyl peroxide (DBPO) or the water soluble 2,2′-azobis(2-methylpropionamidine) dihydrochloride were used (data and chromatograms not shown).| Entry | Solvent | Concentration [mol L−1] | T [°C] | Time [h] | Mn [kDa] | PDI | DP |
|---|---|---|---|---|---|---|---|
| 1 | Dioxane | 0.45 | 100 | 24 | 15 | 1.5 | 70 |
| 2 | NMP | 0.45 | 150 | 24 | 16 | 1.5 | 70 |
| 3 | THF | 0.45 | 60 | 24 | 18 | 1.6 | 90 |
| 4 | — | Bulk | 80 | 8 | 400 | 1.3 | 1800 |
| 25 | 4.6 | 115 | |||||
| 5 | — | Bulk | 80 | 24 | 200 | 5.6 | 900 |
| 6 | — | Bulk | 80–150 | 24 | 550 | 2.6 | 2500 |
Benefiting from the low melting point of M1 (mp = 55 °C), a solventless free radical polymerisation was tested. For this purpose, M1 was melted in a dried tube and the melt was stirred continuously. Then, the radical starter was added and the stirring continued for 8 h. In this fashion, we obtained in a first attempt a polymer that showed a bimodal distribution in the GPC chromatogram with molecular weights of 400 kDa and 25 kDa, respectively (Fig. 1, green curve). The promising formation of polymers with longer chains using this method prompted us to test other conditions in order to obtain monomodal polymers with high molecular weights. In another attempt, simple elongation of the reaction time led to a polymer with a monomodal distribution in the GPC chromatogram with approx. 200 kDa (blue curve).
Along with the variation of reaction times a gradual increase of the temperature from 80 °C to 150 °C during the polymerisation reaction was tested as well. Much to our satisfaction, polymers with molecular weights of around 550 kDa and a polydispersity index (PDI) of 2.6 were obtained (Fig. 1, pink curve). Triggered by the obtained results, reaction times of longer than 24 h were also investigated; however, no significant improvement in obtaining longer chains was observed. It is noteworthy that upon repeating the synthesis of P1, both the solventless as well as the solution-based polymerisations gave reproducible results.
The obtained polymers were characterised by 1H NMR spectroscopy. Fig. 2 shows both the 1H NMR spectra of the monomer M1 and the corresponding polymer P1. The spectrum of the monomer M1 (top) displays the aromatic signals of the protons of the phenyl ring at 7.93 ppm and 8.24 ppm, respectively. In addition to the signals for the methyl ester groups at 3.94 ppm and 3.92 ppm, two doublets can be observed at 5.43 ppm and 5.76 ppm, respectively, as well as a quartet at 7.41 ppm that correspond to the vinylic protons. These proton signals are undoubtedly missing in the 1H NMR spectrum of the polymer P1 indicating the reaction of the vinylic group (Fig. 2, bottom). In addition, a broad signal at 1.80–1.25 ppm is observed that corresponds to the protons of the aliphatic chain. It is also interesting to notice that the methyl ester signals are much more separated in the polymer NMR (3.82 ppm and 3.24 ppm) compared to the monomer, supposedly because of the closer vicinity of the alkyl chain to the carboxylate groups. At the same time, the aromatic signals are merged into each other and give a broad signal centred at 7.46 ppm.
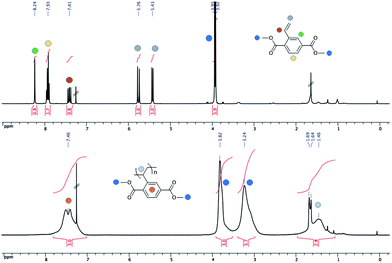 | ||
| Fig. 2 1H NMR spectra (400 MHz, CDCl3) of the monomer M1 (top) and the corresponding polymer P1 (bottom). | ||
Post-functionalisation of polymer P1
For the synthesis of the Li-salt P1-Li that would serve as battery material, the esters of the polymer P1 were first hydrolysed to the corresponding carboxylic acids. After deprotonation, Li+-ions were inserted via two synthetic routes (Scheme 2). The first route (Scheme 2, Route A) follows a literature-known procedure,16 where the hydrolysis of the ester groups and the insertion of the Li+-ions occur in one step by using LiOH. For this purpose, the polymer was dissolved in THF and LiOH was added to the solution. After 12 h reaction time, a precipitate was observed indicating the formation of the polymer Li-salt P1-Li. As the product is well soluble in water, but shows low solubility in THF, the water phase was dropped into an excess amount of THF. The obtained precipitate was subjected to centrifugation and collected by decantation. Since LiOH is soluble in a certain amount of water as well, this procedure was repeated several times in order to obtain the pure polymer P1-Li.To ensure the purity of the material and to synthesise the Li-salt in a more convenient way, another route was sought (Scheme 2, Route B). Following a two-step synthesis, the ester polymer P1 was first hydrolysed with NaOH in a THF/MeOH mixture. Under these conditions, the desired polymer with two carboxylic acid groups P1-COOH precipitated from the solution. The pure compound was obtained after an excessive wash of the precipitate with MeOH in a yield of 80%. Treatment of P1-COOH with LiHMDS in dry THF at 50 °C overnight led to the formation of the target P1-Li. The obtained material was washed excessively with hot THF in order to get rid of the formed by-products.
To validate the insertion of the Li+-ions, the two polymers P1 and P1-Li were characterised by IR-spectroscopy (Fig. 3). The polymer P1 showed two characteristic bands at 1723 cm−1 and 1286 cm−1 that correspond to the stretching vibration of the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and the alkyl groups of the polymer backbone (C–C), respectively. These two bands are markedly shifted in case of the polymer lithium salt P1-Li. The carbonyl band is shifted by about 200 cm−1 to lower wavenumbers and can be observed at 1517 cm−1, while the band for the C–C-bond vibrations are shifted by about 150 cm−1 to higher wavenumbers and can be observed at 1428 cm−1. These significant shifts indicate the change of the vibrational character in the two materials. Moreover, a significant band can be additionally observed at approximately 500 cm−1 in the IR spectrum of P1-Li corresponding to the Li–O deformation vibration, which confirms the successful substitution reaction of the Li+-ions. This band is clearly missing in the ester-functionalised polymer P1.
O) and the alkyl groups of the polymer backbone (C–C), respectively. These two bands are markedly shifted in case of the polymer lithium salt P1-Li. The carbonyl band is shifted by about 200 cm−1 to lower wavenumbers and can be observed at 1517 cm−1, while the band for the C–C-bond vibrations are shifted by about 150 cm−1 to higher wavenumbers and can be observed at 1428 cm−1. These significant shifts indicate the change of the vibrational character in the two materials. Moreover, a significant band can be additionally observed at approximately 500 cm−1 in the IR spectrum of P1-Li corresponding to the Li–O deformation vibration, which confirms the successful substitution reaction of the Li+-ions. This band is clearly missing in the ester-functionalised polymer P1.
 | ||
| Fig. 3 IR-spectroscopic analyses of the ester polymer P1 (top) and the Li-salt polymer P1-Li (bottom). | ||
Electrochemical characterisation of M1 and P1 in solution
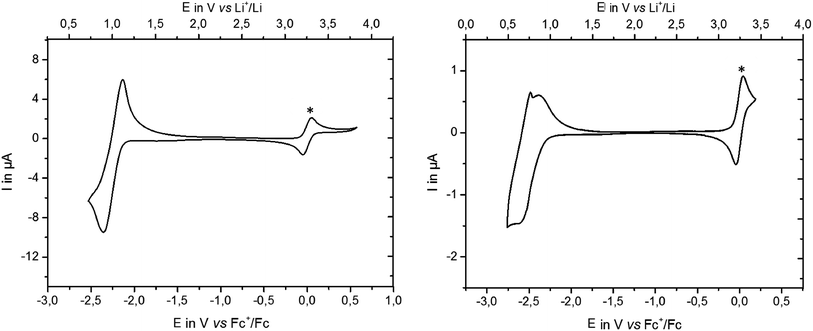
Monomer M1 and the corresponding polymer P1 were characterised electrochemically by means of cyclic voltammetry (CV). The measurements were performed in a 0.1 M solution of the compound in THF with n-tetrabutylammonium hexafluoro-phosphate (TBAPF6) as electrolyte. The cyclic voltammogram of the monomer M1 (Fig. 4, left) showed a reversible reduction wave at −2.35 V vs. Fc+/Fc (∼0.9 V vs. Li+/Li) with two electrons involved referring to the reduction of the methyl esters. In case of the polymer P1 (Fig. 4, right), the cyclic voltammogram showed a reduction process at −2.53 V vs. Fc+/Fc (∼0.72 V vs. Li+/Li) which is about 300 mV cathodically shifted compared to the monomer. The observed redox process showed a broad wave for the reduction which is common for polymers. The re-oxidation of the same polymer showed an impetuous drop of current at −2.49 V vs. Fc+/Fc that recovers again and continues giving a broad wave. Assuming that the polymer exists as a coil, this observation may be related to the faster re-oxidation of the outer units of the polymer due to their easier accessibility while that of the units inside the coil needs more time because of the diffusion dependency.Measurements on polymers with different molecular weights of 18 kDa and 200 kDa led to the same observation (see ESI,† Fig. 2). A similar electrochemical investigation of the Li-salt P1-Li was not possible because of the insolubility of the material in any common organic solvent. Nevertheless, considering the structural similarity of the Li-salt and the methyl ester derivative, the reduction of M1 as well as of P1 at low potentials underlines the principal suitability of this class of compounds as anode material in batteries. It is worth emphasising that the polymer salt P1-Li is completely insoluble in any organic solvent. In particular, the insolubility in common battery electrolytes such as ethylene carbonate/dimethyl carbonate underlines the superiority of polymeric materials in terms of cycling stability in comparison with the corresponding monomers, which is discussed below.
Electrochemical investigations of P1-Li as anode material for organic batteries
The CV of the polymeric anode material P1-Li shows a redox range of 0.5–1.2 V vs. Li+/Li (Fig. 5a) (consistent with the results of P1 in Fig. 4). Only few organic compounds have been described which work at such low potentials upon insertion/de-insertion mechanism, and almost all of them are of low molecular weight.16,26 One example of a polymeric compound is diethyl terephthalate-functionalised polythiophenes, which have been reported recently27-unfortunately no specific capacities are given. P1-Li is another polymer active in this potential region. Generally, a low anode potential is the key to obtaining large voltages and high energies of the full cell.On repeated cycling there is a major change taking place between the first and the second CV cycle, whereas from the second cycle onwards the CV curves change only little. Obviously, some kind of formation process takes place during the first charge, giving rise to significant irreversible capacity.
The galvanostatic charge–discharge profile of P1-Li shown in Fig. 5b (blue curve) confirms the high irreversible loss in the 1st cycle. One reason is the high amount of carbon black (33 wt%) in the electrode, which is indispensable for sufficient conductivity within the electrode, because this kind of active materials is usually non-conductive. It is known that carbon black itself exhibits a large irreversible capacity below 1 V vs. Li+/Li caused by its high surface area and functionalities, which offer many possibilities for reductive side reactions.28,29 These side reactions are visualised in the CV (Fig. 5a, black curve) by the increased reductive currents during the 1st cycle.
The reversible capacity amounts to 58 mA h g−1 (at the C/3 rate used for the galvanostatic cycling test), which is 22% of the theoretical capacity of P1-Li (263 mA h g−1) (Fig. 5b, blue curve). Apparently not all of the material takes part in the electrochemical reaction. A possible reason could be an insufficient electronic conductivity within the electrode. As P1-Li is an electronic insulator, only those areas of the material will electrochemically react which are in contact with the carbon black. Especially for large particles the reaction may therefore be limited to the particle surface. We expect the reversible capacity to increase by decreasing the particle size and by optimising the distribution of and contact between the carbon black and the active material. Generally, literature-known polymers which are used as cathode materials often exhibit lower specific capacities than the calculated theoretical capacities of their particular units.30,31
To test the effect of immobilising the redox-active unit in a polymer, we compared the electrochemical performance of P1-Li to that of the related literature-known monomeric dilithium terephthalate. Dilithium terephthalate was synthesised as previously described,16 and processed into electrodes and tested under the same preparation and cycling conditions as P1-Li. The CV measurements (see ESI,† Fig. 3) indicate higher capacities than for the polymer P1-Li, and a significant irreversible capacity. This is reflected in the galvanostatic measurements (Fig. 5b). Fig. 5b shows, furthermore, that although the polymer (blue) provides a lower initial specific capacity than dilithium terephthalate (red), its cycling stability is significantly improved, resulting in much slower capacity decay. The ratio of the discharge capacities of the 100th cycle to that of the 1st cycle is 85% for P1-Li and only 40% for dilithium terephthalate. The inferior cycling stability of the low-molecular-weight terephthalate is presumably associated with the dissolution of the active material into the electrolyte.16 As well swelling of the material/electrode due to uptake of solvent from the electrolyte may contribute to the capacity fade via loss of electronic contact within the electrode material. In the case of P1-Li the corresponding active units are incorporated into a polymer backbone preventing their dissolution. Thus, regarding long term cycling the polymer P1-Li seems to be the more suitable candidate for battery applications. Last but not least, the polymer P1-Li shows a better rate capability than monomeric dilithium terephthalate (see ESI,† Fig. 4).
Conclusions
The monomer dimethyl 2-vinylterephthalate M1, which was synthesised in this work, exhibits a vinyl group as a polymerisable unit while the carboxylate moieties serve as cation coordinating sites. Synthesis of M1 was performed via three different routes in order to evaluate the route with the highest overall yield. However, in our hands the route with the highest number of synthetic steps gave the highest overall yield. Different conditions were evaluated to obtain polymers of M1 via free radical polymerisation. Solution-based polymerisation with different solvents gave polymers with merely low molecular weights of 18 kDa in average. Taking advantage of the low melting point of M1 it was possible to perform the polymerisation reaction in the bulk. Under these conditions polymer P1 with high molecular weights of 550 kDa could be obtained with reaction times of 24 h and reaction temperatures of approx. 150 °C. With the aim to use polymers as electroactive materials in organic batteries, P1 was post-functionalised to the corresponding lithium salt P1-Li via two different routes. The route with stepwise hydrolysis and Li-insertion using LiHMDS was more convenient in terms of better yields and sophisticated purification.P1-Li was investigated electrochemically using cyclic voltammetry and galvanostatic measurements. The CVs of P1-Li showed redox activity in a potential range of 0.5–1.2 V vs. Li+/Li which assigns the material as a candidate for anode. P1-Li showed high irreversible losses in the first cycle, which is symptomatic for most organic materials. Galvanostatic charge–discharge cycling reveals a specific capacity of 58 mA h g−1 for P1-Li (at a C/3 rate) which is only 22% of the theoretical capacity. However, the polymer displays a good cycling stability, still showing a discharge capacity of 85% after 100 cycles. Especially the comparison with the parent monomer dilithium terephthalate with only 40% discharge capacity after 100 cycles reveals the superiority of the polymer in terms of cycling stability.
This class of polymers seems to be promising for application as anode material in battery applications. Nonetheless, further investigations on how to reduce the irreversible capacity (especially in the first cycle) and on how to increase the practical reversible capacity are needed before it can be used in commercial cells.
Acknowledgements
The authors are indebted to the German Federal Ministry of Education and Research (BMBF) for financial support within the project “LiEcoSafe” (contract no. 03X4636A/B). Dr Günther Götz and Dr Elena Mena-Osteritz (Ulm University) are sincerely acknowledged for fruitful discussions, and Dagmar Weirather-Köstner (ZSW) for complementary electrochemical measurements. The authors thank Zeynab Nikpoor for the graphical illustration of the TOC figure.References
- Y. Liang, Z. Tao and J. Chen, Adv. Energy Mater., 2012, 2, 742 CrossRef CAS.
- Z. P. Song and H. S. Zhou, Energy Environ. Sci., 2013, 6, 2280 CAS.
- P. Poizot and F. Dolhem, Energy Environ. Sci., 2011, 4, 2003 CAS.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed.
- K. Sakaushi, E. Hosono, G. Nickerl, T. Gemming, H. Zhou, S. Kaskel and J. Eckert, Nat. Commun., 2013, 4, 1485 CrossRef PubMed.
- L. Zhan, Z. Song, J. Zhang, J. Tang, H. Zhan, Y. Zhou and C. Zhan, Electrochim. Acta, 2008, 53, 8319 CrossRef CAS.
- L. Zhan, Z. Song, N. Shan, J. Zhang, J. Tang, H. Zhan, Y. Zhou, Z. Li and C. Zhan, J. Power Sources, 2009, 193, 859 CrossRef CAS.
- N. Oyama, T. Tatsuma, T. Sato and T. Sotomura, Nature, 1995, 373, 598 CrossRef CAS.
- S. R. Deng, L.-B. Kong, G.-Q. Hu, T. Wu, D. Li, Y.-H. Zhou and Z.-Y. Li, Electrochim. Acta, 2006, 51, 2589 CrossRef CAS.
- T. Janoschka, M. D. Hager and U. S. Schubert, Adv. Mater., 2012, 24, 6397 CrossRef CAS PubMed.
- T. Jähnert, M. D. Hager and U. S. Schubert, J. Mater. Chem. A, 2014, 2, 15234 Search PubMed.
- K. Oyaizu and H. Nishide, Adv. Mater., 2009, 21, 2339 CrossRef CAS.
- K. Nakahara, K. Oyaizu and H. Nishide, Chem. Lett., 2011, 40, 222 CrossRef CAS.
- P. Novák, K. Müller, K. S. V. Santhanam and O. Haas, Chem. Rev., 1997, 97, 207 CrossRef.
- B. Häupler, A. Wild and U. S. Schubert, Adv. Energy Mater., 2015, 5, 1402034 CrossRef.
- M. Armand, S. Grugeon, H. Vezin, S. Laruelle, P. Ribiére, P. Poizot and J.-M. Tarascon, Nat. Mater., 2009, 8, 120 CrossRef CAS PubMed.
- L. M. Zhu, W. Shi, R. R. Zhao, Y. L. Cao, P. X. Ai, A. W. Lei and H. X. Yang, J. Electroanal. Chem., 2013, 688, 118 CrossRef CAS.
- J. Heinze, B. A. Frontana-Uribe and S. Ludwigs, Chem. Rev., 2010, 110, 4724 CrossRef CAS PubMed.
- Z. Song, Y. Qian, X. Liu, T. Zhang, Y. Zhu, H. Yu, M. Otani and H. Zhou, Energy Environ. Sci., 2014, 7, 4077 CAS.
- Z. Song, Y. Qian, T. Zhang, M. Otani and H. Zhou, Adv. Sci., 2015, 2, 1500124 CrossRef.
- Z. Song, Y. Qian, M. L. Gordin, D. Tang, T. Xu, M. Otani, H. Zhan, H. Zhou and D. Wang, Angew. Chem., Int. Ed., 2015, 54, 13947 CrossRef CAS PubMed.
- L. Chen, W. Li, Y. Wang and Y. Xia, RSC Adv., 2014, 4, 25369 RSC.
- W. W. Deng, S. M. Liang, X. Y. Wu, J. F. Qian, Y. L. Cao, X. P. Ai, J. W. Feng and H. X. Yang, Sci. Rep., 2013, 3, 2671 Search PubMed.
- H. Qin, Z. P. Song, H. Zhan and Y. H. Zhou, J. Power Sources, 2014, 249, 367 CrossRef CAS.
- G. Wittig and U. Schöllkopf, Chem. Ber., 1954, 87, 1318 CrossRef.
- (a) W. Walker, S. Grugeon, H. Vezin, S. Laruelle, M. Armand, F. Wudl and J.-M. Tarascon, J. Mater. Chem., 2011, 21, 1615 RSC; (b) T. Yasuda and N. Ogihara, Chem. Commun., 2014, 50, 11565 RSC.
- L. Yang, X. Huang, A. Gogoll, M. Strømme and M. Sjödin, Electrochim. Acta, 2016, 204, 270 CrossRef CAS.
- L. Fransson, T. Eriksson, K. Edström, T. Gustafsson and J. O. Thomas, J. Power Sources, 2001, 101, 1 CrossRef CAS.
- K. Takei, N. Terada, K. Kumai, T. Iwahori, T. Uwai and T. Miura, J. Power Sources, 1995, 55, 191 CrossRef CAS.
- J. Xiang, C. Chang, M. Li, S. Wu, L. Yuan and J. Sun, Cryst. Growth Des., 2008, 8, 280 CAS.
- T. L. Gall, K. H. Reiman, M. C. Grossel and J. R. Owen, J. Power Sources, 2003, 119–121, 316 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra24064j |
| This journal is © The Royal Society of Chemistry 2016 |