Triblock and pentablock copolymerizations of ε-caprolactone with l-lactide catalyzed by N-heterocyclic carbene†
Abstract
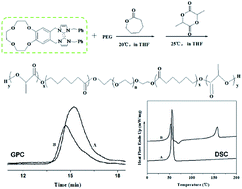
Dihydroxyl capped biodegradable poly(ε-caprolactone)–poly(ethylene glycol)–poly(ε-caprolactone) (PCL–PEG–PCL) triblock copolymer and poly(L-lactide)–poly(ε-caprolactone)–poly(ethylene glycol)–poly(ε-caprolactone)–poly(L-lactide) (PLLA–PCL–PEG–PCL–PLLA) pentablock copolymer have been synthesized by using benzo-12-crown-4-imidazole carbene (B-12-C-4imY) as a catalyst, and PEG and PCL–PEG–PCL copolymer as macroinitiators, respectively. We focused on the influence of the varied reaction time, temperature and monomer/catalyst molar ratio on the copolymerizations. Chemical structures and physical properties of the block copolymers were studied through the characterization of 1H, 13C NMR, GPC and DSC. The NMR spectral data of the copolymers displayed the chemical structures without random placement. Therefore, the pure triblock and pentablock copolymers have been synthesized successfully.

 Please wait while we load your content...
Please wait while we load your content...