Hyalodendriellins A–F, new 14-membered resorcylic acid lactones from the endophytic fungus Hyalodendriella sp. Ponipodef12†
Abstract
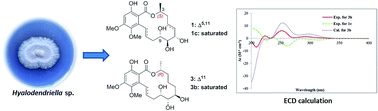
Six new 14-membered resorcylic acid lactones (RALs), named hyalodendriellins A–F (1–6), were isolated from the culture of the endophytic fungus Hyalodendriella sp. Ponipodef12 associated with the hybrid ‘Neva’ of Populus deltoides Marsh × P. nigra L. The structures of the new compounds were deduced by analyses of the NMR spectroscopic and mass spectrometry data, in combination with chemical conversion, modified Mosher's method and TDDFT ECD calculations for determining the absolute configurations. Compounds 1, 2, 4, and 6 possess a 3S configuration, while 3 and 5 are 3R-configurated. The co-occurrence of RALs with different stereochemistry at C-3 in the same fungus is rare. The CD behaviors of 1–6 as well as their acetonide and hydrogenated derivatives were investigated. All the isolated compounds were evaluated for their antinematodal, larvicidal, cytotoxic, antibacterial and antifungal activities. Among them, hyalodendriellin A (1) displayed moderate antinematodal activity against Caenorhabditis elegans and Meloidogyne incognita. Hyalodendriellin C (3) exhibited larvicidal effect against the fourth-instar larvae of the mosquito Aedes aegypti.

 Please wait while we load your content...
Please wait while we load your content...