A new approach designed for improving flame retardancy of intumescent polypropylene via continuous extrusion with supercritical CO2
Pengke Huangab,
Yongyan Pang *a,
Lihua Zhanga,
Fei Wua,
Shuhai Zhangb and
Wenge Zheng*a
*a,
Lihua Zhanga,
Fei Wua,
Shuhai Zhangb and
Wenge Zheng*a
aNingbo Key Laboratory of Polymer Materials, Polymers and Composites Division, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, 1219 Zhongguan West Road, Ningbo, Zhejiang Province 315201, China. E-mail: yongyan.pang@nimte.ac.cn; wgzheng@nimte.ac.cn
bSchool of Chemical and Environmental Engineering, North University of China, Taiyuan, Shanxi Province 030051, China
First published on 23rd November 2016
Abstract
The objective of this study was to explore a new approach to enhance the flame retardancy of polypropylene/intumescent flame retardant (PP/IFR) composites through improving IFR dispersion via continuous extrusion with supercritical carbon dioxide (scCO2) as the processing medium. The IFR used in this study consisted of ammonium polyphosphate (APP) and pentaerythritol (PER) at a weight ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Scanning electron microscopy (SEM) was applied to study the dispersion of IFR in the PP composites prepared in the presence of different contents of scCO2. Thermogravimetric analysis (TGA) was carried out to study the effect of IFR dispersion on the thermal stability and the yield of char residues of the PP composites. A plastic smoke generation tester was applied to study the specific optical density under a thermal irradiance. SEM was then used to study the morphologies of the intumescent char formed after the smoke testing. Limiting oxygen index (LOI) and vertical burning test (UL-94) ratings were evaluated to study the effect of IFR dispersion on the flame retardancy of the PP composites. It was found that scCO2 as the processing medium could significantly improve IFR dispersion and thus increase the efficiency of the flame retardant and enhance the flame retardancy of the PP composites. This new approach could allow PP composites to pass the UL-94 V-0 rating at a lower IFR content. Possible mechanisms were proposed for the scCO2-aided IFR dispersion and the improved flame retardancy of the PP composites.
1. Scanning electron microscopy (SEM) was applied to study the dispersion of IFR in the PP composites prepared in the presence of different contents of scCO2. Thermogravimetric analysis (TGA) was carried out to study the effect of IFR dispersion on the thermal stability and the yield of char residues of the PP composites. A plastic smoke generation tester was applied to study the specific optical density under a thermal irradiance. SEM was then used to study the morphologies of the intumescent char formed after the smoke testing. Limiting oxygen index (LOI) and vertical burning test (UL-94) ratings were evaluated to study the effect of IFR dispersion on the flame retardancy of the PP composites. It was found that scCO2 as the processing medium could significantly improve IFR dispersion and thus increase the efficiency of the flame retardant and enhance the flame retardancy of the PP composites. This new approach could allow PP composites to pass the UL-94 V-0 rating at a lower IFR content. Possible mechanisms were proposed for the scCO2-aided IFR dispersion and the improved flame retardancy of the PP composites.
1. Introduction
Polypropylene (PP) has been widely used in electrical appliances, automobiles, and buildings due to its good mechanical properties, chemical stability, easy processing and low cost, etc. In the fields above, fire safety regulations and measures are strictly enforced, which require PP to meet certain flame retardant ratings. However, PP shows burning characteristics of easy flammability accompanied by severe dripping, which would cause catastrophic disasters when a fire is initiated.1,2 Hence, flame retardant modification of PP is of great importance to meet the fire safety standards and thus to expand its applications. Brominated flame retardants are traditionally used as additives in PP owing to the high flame retardancy and cost effectiveness. However, in recent years, due to the rising environmental and health concerns, applications of halogen-free flame retardants have been demanded in many sectors.3,4In recent years, intumescent flame retardant (IFR) has been extensively investigated on flame retardant PP due to its low smoke, low toxicity and anti-dripping, etc.5,6 Generally, an IFR system is composed of three parts: an acid source, e.g. ammonium polyphosphate, a charring source, e.g. pentaerythrotol, and a blowing source, e.g. melamine. The flame retarding mechanism of intumescent char formation has been reported for IFR elsewhere.7 During heating, the acid source could decompose to generate inorganic acid, which could dehydrate the charring source in esterification reactions to form carbonaceous char. Subsequently, the gas produced by the blowing source could swell the carbonaceous char to form intumescent char layers, which covered on the sample surface as barriers to protect the underlying materials. However, the dispersion of IFR in PP is usually poor due to the bad compatibility, which results in a low efficiency of the flame retardant. Based on the previous reports,8,9 an addition of more than 30 wt% of IFR is usually required for the PP composites to pass the UL-94 V-0 rating. As such, how to improve the flame retardancy of intumescent PP has been an important and meaningful task. Incorporating synergistic agents has proven one feasible means, which includes metallic compounds, carbon nanotube and layered double hydroxides, etc.10–13
Another approach to enhance the flame retardancy of polymer composites could be through improving the dispersion of flame retardants in the polymer matrix.14–17 Gui et al.14 fabricated a ternary composite of polymer/crosslinked rubber/nano-magnesium hydroxide (MH) via first preparation of a special compound powder of crosslinked rubber/nano-MH by a co-spray technique, and found that the better dispersion of nano-MH in the composite favored the better flame retardancy. Huang et al.15 found that the functionalization of graphene by grafting a synthesized intumescent flame retardant could improve the dispersion of graphene in the polymer matrix and hence improved the flame retardancy of the nanocomposites. Peeterbroeck et al.16 reported that a better dispersion of the high-density polyethylene coated multiwalled carbon nanotubes (c-MWNTs) in EVA relative to that of the uncoated nanotubes could explain the better flame retardancy. Till now, the approaches for improving the dispersion of flame retardants usually involve no less than two steps of synthesis or modification as well as processing. Hence, an efficient and practical approach that could directly process flame retardant polymer composites with good dispersion is still demanded.
Recently, supercritical carbon dioxide (scCO2) has usually applied in synthesis, processing, foaming and particle formation due to its gas-like diffusivity, liquid-like density and mild supercritical condition (31.1 °C and 7.38 MPa) as well as environmental friendliness, chemical inertness and low cost.18–22 According to the previous literature,18–22 scCO2 could lower the viscosity of polymer melts and increase the mass transfer rate due to its plasticizing effect on polymers. Some studies19,22,23 showed that thermal processing in the presence of scCO2 facilitated the intercalation and exfoliation of polymer/clay nanocomposites. It could be partially due to the larger mobility of macromolecules upon the plasticizing effect of scCO2 and partially due to the increase of interlayer space upon the expansion of scCO2 during pressure release. Ma et al.24 reported that the presence of scCO2 as the thermal processing medium favored the dispersion of PP/sepiolite composites. Wu et al.25 proposed an in situ bubble-stretching model to explain the improved dispersion of inorganic additives in polymers. It was believed that the stretching of the surrounding polymers and oscillation during bubble expansion of foaming could facilitate the particle dispersion. Goren et al.26 reported that the foaming with CO2 from supercritical state to gas could break the aggregates of SiO2 and thus improved SiO2 dispersion in polymer matrix. Till now, scCO2 has seldom been applied as a processing medium during thermal processing to improve the dispersion of flame retardants and thus to enhance the flame retardancy. If this could be proved feasible, a new practical and efficient approach could be established for improving the flame retardant properties of intumescent PP. Recently, with utilization of scCO2 as the processing medium or foaming agent, our group has made a series of progresses in improving phase dispersion, mechanical properties, long-chain branching, in situ compatibilization, and fabricating bimodal-cell and open-cell foams.27–32 The current study would further expand the application of scCO2 as a green processing medium to flame retardant materials.
This study aimed to explore a new practical approach to enhance the flame retardancy of PP/IFR composites through improving the dispersion of IFR via a thermal process of continuous extrusion with scCO2 as the processing medium. A traditional IFR system applied was ammonium polyphosphate (APP) and pentaerythritol (PER) at a weight ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Many characterizations were carried out such as scanning electron microscope (SEM), thermogravimetric analysis (TGA), smoke generation, limiting oxygen index (LOI) and UL-94 tests to study the relationship between the IFR dispersion and the flame retardancy of the PP/IFR composites. Possible mechanisms were proposed for the scCO2-aided IFR dispersion and the consequent improved flame retardancy.
1. Many characterizations were carried out such as scanning electron microscope (SEM), thermogravimetric analysis (TGA), smoke generation, limiting oxygen index (LOI) and UL-94 tests to study the relationship between the IFR dispersion and the flame retardancy of the PP/IFR composites. Possible mechanisms were proposed for the scCO2-aided IFR dispersion and the consequent improved flame retardancy.
2. Experimental
2.1. Materials
Polypropylene (PPB-MO2) used in this study was purchased from Sinopec Zhenhai Refining & Chemical Company (China), with an MFI of 1.6 g/10 min at 230 °C/2.16 kg. Ammonium polyphosphate (APP, EPFR-APP231) and pentaerythritol (PER, CAS 115-77-5) were supplied by Polyrocks Chemical co. LTD and Aladdin, respectively. APP and PER were used at a mass ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Carbon dioxide (CO2) with a purity of 99.9% was provided by Ningbo Wanli Gas Corporation.
1. Carbon dioxide (CO2) with a purity of 99.9% was provided by Ningbo Wanli Gas Corporation.
2.2. Samples preparation
PP, APP and PER were vacuum-dried at 60 °C for 8 h before use. The sample preparation included the following four steps: first, PP/APP/PER premix was prepared using a TSE-35A/600-15-48 twin screw extruder (Nanjing Yarui, China) with a screw diameter of 35 mm and a length diameter ratio of 25, with a rotating speed at 200 rpm and a processing temperature at 195 °C; second, the PP/APP/PER premix was fed into a tandem single-screw extruder to prepare the flame retardant PP composites with different contents of scCO2 as a processing medium; third, the PP composites were compression-molded into sheets with a XLB50-D compression molder (Huzhou Shuangli Automation Technology, China) at 190 °C under a pressure of 10 MPa for 5 min; fourth, the sheets were cut into standard samples for different tests. It is noted that scCO2 was used only as a processing medium for promoting the dispersion of IFR in PP and could easily totally be removed during the compression molding. The formulations of the PP/IFR composites are shown in Table 1.| Samples | Composition of PP/IFR | scCO2 (wt%) | ||
|---|---|---|---|---|
| PP | APP | PER | ||
| PP/23IFR/0scCO2 | 77.0 | 17.3 | 5.7 | 0 |
| PP/23IFR/3scCO2 | 77.0 | 17.3 | 5.7 | 3 |
| PP/23IFR/5scCO2 | 77.0 | 17.3 | 5.7 | 5 |
| PP/23IFR/7scCO2 | 77.0 | 17.3 | 5.7 | 7 |
| PP/25IFR/0scCO2 | 75.0 | 18.7 | 6.3 | 0 |
| PP/25IFR/3scCO2 | 75.0 | 18.7 | 6.3 | 3 |
| PP/25IFR/5scCO2 | 75.0 | 18.7 | 6.3 | 5 |
| PP/25IFR/7scCO2 | 75.0 | 18.7 | 6.3 | 7 |
| PP/30IFR/0scCO2 | 70.0 | 22.5 | 7.5 | 0 |
| PP/30IFR/3scCO2 | 70.0 | 22.5 | 7.5 | 3 |
| PP/30IFR/5scCO2 | 70.0 | 22.5 | 7.5 | 5 |
| PP/30IFR/7scCO2 | 70.0 | 22.5 | 7.5 | 7 |
The description about the tandem extrusion system used in this study can be found in the previous literature.27–32 It mainly consists of two extruders, two drive motors, an ISCO syringe pump, a gear pump, a heat exchanger and a nozzle, etc. There are several thermocouples and pressure transducers located in the two extruders and the nozzle, which can provide the temperature and pressure online during the process. The scCO2 was injected at the three quarter point of the first extruder by the syringe pump under a pressure of 18 MPa. The concentration of scCO2 was regulated by adjusting its flow rate through the syringe pump referred to the materials feed rate31,33 and was kept at the ratio of 0 wt%, 3 wt%, 5 wt% and 7 wt% of the mixture, respectively. The first extruder was used to plasticize the polymers and preliminarily mix the scCO2 with the intumescent flame retardants and polymers, with a rotating speed at 36 rpm and a processing temperature at 200 °C. The second extruder was used to completely and uniformly dissolve the scCO2 in the polymer melts, with a rotating speed at 5 rpm and a processing temperature set at 190 °C. The pressure of the whole system was ca. 10–15 MPa. The processing condition in this study was above the supercritical condition of CO2 (31.1 °C and 7.38 MPa), and thereby, it was guaranteed that CO2 was in supercritical state during the process.
Actually, based on the previous literature,34 the concentration of scCO2 used in this study was below its solubility in PP at the processing condition to make sure that it can completely dissolve in PP melts. In addition, according to Park et al.35 and Ohshima et al.,36 the diffusivity of scCO2 in PP at 188 °C and 200 °C was ca. 4.2 × 10−5 cm2 s−1 and 8.0 × 10−5 cm2 s−1, respectively. Upon the injection of scCO2 into the polymer melts, it took only several seconds for scCO2 to be completely dissolved in PP melts and formed a one-phase polymer/scCO2 solution. In this study, the mixing and residence time for scCO2 in the extruders was ca. 15 min. Thereby, scCO2 was definitely able to be completely and uniformly dissolved in PP melts, which was considered favorable for improving the dispersion of IFR in PP.
2.3. Thermogravimetry analysis (TGA)
Thermogravimetry analysis of the PP/IFR composites was carried out with a Mettler Toledo TGA/DSC1 thermal analyser from 50 °C to 800 °C at a heating rate of 10 °C min in nitrogen atmosphere with a flow rate of 50 ml min−1. A calculated TGA curve was obtained for PP/25IFR composite in the same temperature range, assuming that no interaction happened among PP, APP and PER. The calculated curve was generated based on the eqn (1), which was the summation of the TGA curves of each individual component in the mixture multiplied by their weight fractions:37| M = 0.75mPP + 0.25(0.75mAPP + 0.25mPER) | (1) |
2.4. Limiting oxygen index (LOI) measurement
The limiting oxygen index values were measured with a Vouch 5801A digital oxygen index tester (Suzhou Yangyi, China) according to GB/T 2406.2-2009. The specimens used were 100 × 6.5 × 3.2 mm3 in dimensions.2.5. Vertical burning (UL-94) testing
The vertical burning test was carried out with a Vouch 5402 H-V burning tester (Suzhou Yangyi, China) according to GB/T2408-2008. The specimens used were 100 × 13 × 3.2 mm3 in dimensions.2.6. Smoke density testing
The smoke density test was carried out with a Vouch 5920 plastic smoke generation tester (Suzhou Yangyi, China) according to GB/T8323.2-2008. Sheet samples with dimensions of 70 × 70 × 1 mm3 were placed on a metallic grid under an irradiance of 50 kW m−2. The optical density was measured continuously with an optical system. By measuring the light transmission, the specific optical density (Ds) was obtained based on eqn (2):38| Ds(t) = 132 × log(100/T(t)) | (2) |
2.7. Scanning electron microscope (SEM) observation
The effect of scCO2 as a processing medium on the dispersion of IFR in PP was investigated with a Zesis EVO18 scanning electron microscope at an acceleration voltage of 20 kV after the specimens were freeze-fractured in liquid nitrogen and a layer of gold was sputtered on the surface. Morphologies of the intumescent char layers of the PP composites after smoke density testing was also characterized with SEM.3. Results and discussion
3.1. Effect of scCO2 on IFR dispersion
The effect of scCO2 as a processing medium on the dispersion of the flame retardants in PP/25IFR composites was investigated with SEM and shown in Fig. 1 (two magnifications). For PP/25IFR/0scCO2, big IFR agglomerates were observed in PP, indicating bad dispersion of IFR in the polymer matrix at a relatively higher content. For PP/25IFR/3scCO2, the IFR agglomerates were decreased in size. For PP/25IFR/5scCO2 and PP/25IFR/7scCO2, the dispersion of IFR was even better. The results indicated that scCO2 as a processing medium had a positive effect on the dispersion improvement of IFR.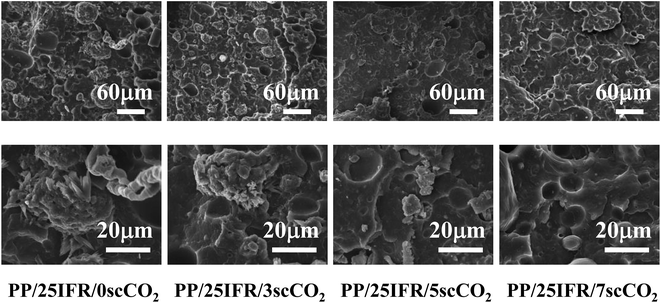 | ||
| Fig. 1 SEM images of the dispersion of flame retardants in PP/25IFR composites prepared with different contents of scCO2. | ||
The reason that scCO2 as a processing medium enhanced IFR dispersion could be interpreted based on its effect during polymer processing and upon pressure release as follows. First, numerous studies18–22 have already reported that scCO2 could plasticize polymers and thus lower the viscosity of polymer melts and increase the mass transfer rate. This technology has been applied to in situ polymerization and reactive extrusion, etc. In this study, the scCO2 was completely and homogeneously dissolved in the PP melts, and thereby, it was acceptable to infer that the presence of scCO2 could increase the mass transfer rate of IFR during processing and thus improve the dispersion. Second, Wu et al.25 proposed that the stretching of polymer melts during bubble expansion upon pressure release improved the dispersion of inorganic additives in polymers. Goren et al.26 also reported that the expansion of CO2 during foaming could break the SiO2 aggregates and then improved the dispersion of SiO2. In this study, although the temperature at the die was relatively too high for the polymer melts to hold the gas to be well foamed, the force generated during gas escape upon pressure drop could also help improve the dispersion of IFR in PP.
3.2. Effect of IFR dispersion on thermal stability
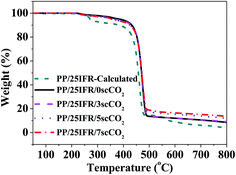
Thermogravimetric analysis was characterized in nitrogen atmosphere to study the thermal stability and the yield of char residues of the PP composites. The TGA curves of the PP/25IFR composites prepared in the presence of different contents of scCO2 and the related data are shown in Fig. 2 and Table 2, respectively. It was found that all the PP/25IFR composites showed similar thermal decomposition behavior. The thermal decomposition occurred in the temperature range of 230–400 °C was mainly due to the decomposition of APP and PER and the subsequent esterification reactions. The thermal decomposition of APP could generate phosphoric acid, which could dehydrate PER in an esterification reaction. The thermal decomposition above 400 °C was mainly about the decomposition of PP and some formed products during the heating.39 A calculated TGA curve was obtained for the PP/25IFR composite, assuming that PP, APP and PER were independent with each other and no interaction occurred during the heating. The inconsistency in TGA curve as well as the yield of the char residues between the experimental and the calculated data confirmed otherwise the interaction among PP, APP and PER during the heating. Thereby, it could imagine that the improved dispersion of IFR could favor their interactions. For the PP/25IFR composites prepared with scCO2, with the increasing content of scCO2, T1 wt% (decomposition temperature at 1 wt%) was decreased, T50 wt% (decomposition temperature at 50 wt%) was increased, and the char residues at 800 °C was increased, indicating that better dispersion of IFR endorsed better thermal stability and larger yield of char residues of the PP/25IFR composites.| Samples | T1 wt% (°C) | T50 wt% (°C) | Residues (800 °C, %) |
|---|---|---|---|
| PP/25IFR-calculated | 239.8 | 458.2 | 4.4 |
| PP/25IFR/0scCO2 | 244.3 | 465.7 | 8.5 |
| PP/25IFR/3scCO2 | 237.3 | 471.0 | 9.0 |
| PP/25IFR/5scCO2 | 234.6 | 471.2 | 11.8 |
| PP/25IFR/7scCO2 | 234.5 | 471.7 | 13.5 |
3.3. Effect of IFR dispersion on smoke generation
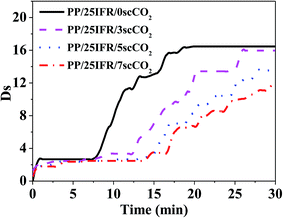
The specific optical density (Ds) can reflect the opacity due to smoke formation, and thus usually be used to evaluate the generation of smoke under heat irradiance. The smoke density curves of the PP/25IFR composites prepared with different contents of scCO2 are shown in Fig. 3. For PP/25IFR/0scCO2, under the thermal irradiance, the specific optical density started to increase rapidly at about 7 min and reached a maximum value of 16.5 at about 16 min. With the increase of the content of scCO2, the specific optical density was retarded to increase, and increased relatively slower to a lower value. The larger char yield favored by the scCO2-aided better dispersion of IFR could well explain the decrease in smoke generation during the heating, as the previous literature38 reported that a higher char yield could contribute to a reduction in smoke density. The result could also be attributed to the fact that IFR could work as smoke suppressants.40,413.4. Effect of IFR dispersion on intumescent char formation
The char quality plays an important role in determining the flame retardancy in a condensed phase action. The formation of an intumescent char layer covering on the whole sample surface could efficiently slow down the transfer of heat and oxygen and protect the underlying polymer matrix from thermal decomposition. Morphologies of the char residues of PP/25IFR composites after the smoke density tests were studied, and the digital photos and the SEM images are shown in Fig. 4. The results showed that for PP/25IFR/0scCO2, the intumescent char layer formed was not in good quality, with incomplete char indicated by an arrow in the digital photo and collapse and cracks in the char in the SEM images. For PP/25IFR/3scCO2, the char quality was improved relatively, but the defects and incompleteness were still observed. For PP/25IFR/5scCO2 and PP/25IFR/7scCO2, intumescent char layers were observed covering the whole sample surface. The results indicated that the scCO2-aided better dispersion of IFR facilitated formation of char layers in better quality. Hence, it was highly expected that the PP/25IFR composites prepared with 5 wt% and 7 wt% scCO2 would show better flame retardancy compared to those prepared with 3 wt% and 0 wt% scCO2.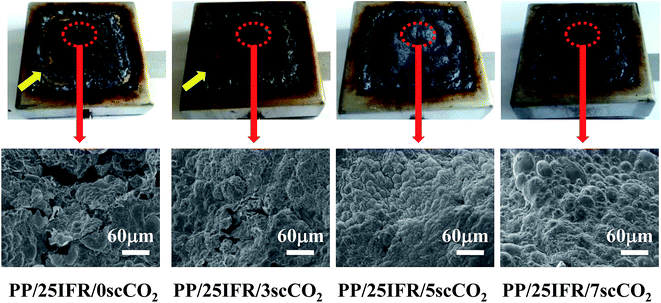 | ||
| Fig. 4 Digital photos and SEM images of morphologies of the char residues of PP/25IFR composites prepared with different contents of scCO2. | ||
3.5. Effect of IFR dispersion on flame retardancy
The effect of scCO2-aided IFR dispersion on the flame retardancy of PP/25IFR composites was studied with the limited oxygen index and vertical burning tests (Table 3). With the increase of scCO2, LOI values were increased, indicating that the minimum concentration of oxygen required for combustion in the mixture of oxygen and nitrogen was increased. Also, with the increase of scCO2, the UL-94 rating was increased and the dripping was effectively suppressed. To be in details, PP/25IFR/0scCO2 could only pass the UL-94 V-2 rating, while PP/25IFR/3scCO2 passed the V-1 rating, and PP/25IFR/5scCO2 and PP/25IFR/7scCO2 passed the V-0 rating. These results demonstrated that the better dispersion of IFR facilitated the improvement of flame retardancy of the PP/25IFR composites. The findings were consistent with the previous reports documenting that the improved the dispersion of nano-MH, graphene and multiwalled carbon nanotubes could endorsed better flame retardancy of the polymer composites.14–163.6. Effectiveness of scCO2-aided improvement of flame retardancy of PP/IFR composites
The results above showed that scCO2 as a processing medium could improve IFR dispersion in PP matrix and thus enhanced the flame retardancy of PP/25IFR composites. It was considered that if through using scCO2 to improve the dispersion of IFR in PP a lower loading of IFR could allow the PP composite to pass UL-94 V-0 rating, less IFR additives would then be required for the new processing approach. Thereby, PP/23IFR and PP/30IFR composites were prepared in the presence of different contents of scCO2 as well as PP/25IFR composites to verify the predication above and the feasibility of the new approach.The char residues of the various PP/IFR composites at 800 °C after TGA tests as a function of scCO2 are shown in Fig. 5. The residues of PP/23IFR, PP/25IFR and PP/30IFR composites were all increased with scCO2, indicating that the scCO2-aided IFR dispersion facilitated the yield of char residues of all the PP composites, which would favor the improvement of flame retardancy.
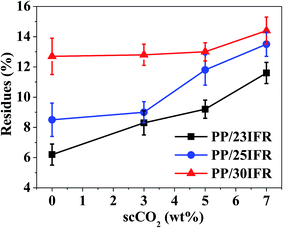 | ||
| Fig. 5 The effect of the contents of scCO2 on the yield of char residues of various PP/IFR composites at 800 °C after TGA tests. | ||
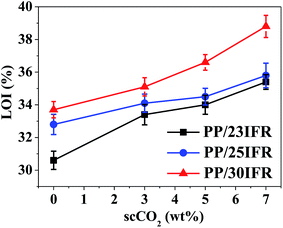
The LOI values of the various PP/IFR composites as a function of scCO2 are shown in Fig. 6. It was found that the LOI values of PP/23IFR, PP/25IFR and PP/30IFR composites were all increased with scCO2, revealing that the improved IFR dispersion increased the oxygen percentage required to support the combustion. It was also observed that with more contents of scCO2 as the processing medium, the PP/23IFR composite had a LOI value as high as that of PP/30IFR prepared with no or less contents of scCO2. Similar findings were also observed in Table 4 about the UL-94 ratings for the PP/IFR composites. With 5 wt% or 7 wt% scCO2 as the processing medium, PP/23IFR passed the UL-94 V-0 rating that PP/30IFR could pass without scCO2. It meant that in this study, with scCO2 as the processing medium, addition of 23 wt% IFR allowed the PP composite to pass the UL-94 V-0 rating, and such a low loading required of IFR has seldom been reported in the previous literature without incorporating a synergistic agent. A previous study42 reported that the online application of ultrasound to a static mixer die coupled to a single-screw extruder improved the dispersion of IFR and hence enhanced the flame retardancy of PP composites. In their study, the formulation of 7 wt% APP, 7 wt% PER and 7 wt% melamine combined with 1 wt% clay and 2 wt% anhydride-grafted PP allowed the PP composite to pass the UL-94 V-0 rating. The low loading of 21 wt% IFR required in their study could be attributed to the improved dispersion of both the IFR additives and clay, and also the synergistic effect between them under the heating. In this study, without incorporating any synergistic agent, only through utilizing scCO2 as the processing medium to improve IFR dispersion in PP could significantly increase the efficiency of the flame retardant and allowed the PP composite to pass the UL-94 rating at a low loading of 23 wt% IFR. Thus, it has demonstrated a feasible approach to fabricate high flame retardant PP/IFR composites through improving the dispersion of IFR with scCO2 as a processing medium, and this new approach could probably be applied to many other flame retardant systems.
| Samples | scCO2 (wt%) | |||
|---|---|---|---|---|
| 0 | 3 | 5 | 7 | |
| PP/23IFR | NO | V-2 | V-0 | V-0 |
| PP/25IFR | V-2 | V-1 | V-0 | V-0 |
| PP/30IFR | V-0 | V-0 | V-0 | V-0 |
3.7. Possible mechanisms proposed for scCO2-aided IFR dispersion and improved flame retardancy
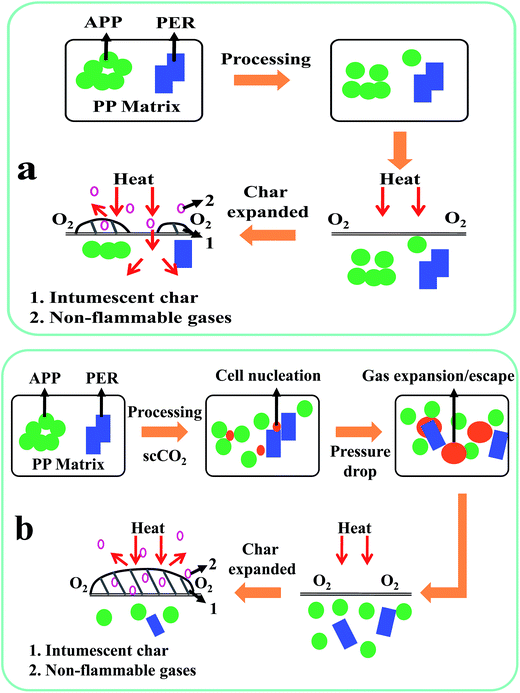
To help more clearly understand the improved IFR dispersion and the enhanced flame retardancy of the PP/IFR composites prepared in the presence of scCO2 as the processing medium, possible mechanisms were proposed. The schematics in Fig. 7a (as comparison) and Fig. 7b were illustrated for PP/IFR composites prepared without and with scCO2, respectively. For PP/IFR composites prepared in the absence of scCO2 (Fig. 7a), big agglomerates would be formed due to the poor compatibility between the flame retardants and the polymer matrix. Under heat flux, no uniform intumescent char layer could be formed covering the whole sample surface due to the bad dispersion of IFR, which thus could not protect the underlying polymer matrix from thermal decomposition and generation of inflammable substances to feed the combustion. In comparison, for PP/IFR composites prepared in the presence of scCO2 (Fig. 7b), the dispersion of IFR could be well improved, possibly partially due to the high mass transfer rates arising from the plasticizing effect of scCO2 on the polymers during thermal processing18–22 and partially due to the in situ stretching effect during bubble expansion25 (at suitable foaming temperatures) or during gas escape (at relatively higher temperatures) upon pressure release. Thereby, under heat flux, a uniform intumescent char layer could be formed covering the whole sample surface, which could keep the heat and oxygen out and prevent the underlying polymer matrix from further thermal decomposition. Hence, better flame retardancy was endorsed by the scCO2-aided IFR dispersion. Due to the complexity arising from too many experimental factors that could affect the processing of polymer composites processing with scCO2 as the processing medium, further studies are still needed to more specifically elucidate the mechanism for the scCO2-aided IFR dispersion and also to explore the optimized processing conditions for improving IFR dispersion in PP.4. Conclusions
This study aimed to explore a new approach to enhance the flame retardancy of PP/IFR composites through improving IFR dispersion via continuous extrusion in the presence of scCO2 as a green processing medium. It was found that the dispersion of IFR was significantly improved with scCO2 and that the improved IFR dispersion endorsed the PP composites better thermal stability, more char residues, better intumescent char layers, less smoke density, and enhanced flame retardancy. The improved IFR dispersion increased the efficiency of the flame retardant and thereby allowed the PP composites to pass UL-94 rating at a relatively lower content of flame retardants. In this study, with 5 wt% scCO2 as the processing medium, PP/23IFR composite passed UL-94 V-0 rating, which usually required a loading of IFR no less than 30 wt% for the conventional processing means. The processing in this study combines the characteristics of capacity of pilot-scale production of continuous extrusion and environmental friendliness, easy handling and removal, and low costs of scCO2. Therefore, it has demonstrated potential to establish a new approach to improve the flame retardancy of PP/IFR composites, and this approach is highly expected to be applied to many other flame retardant systems.Acknowledgements
Financial supports from National Natural Science Foundation of China (51603222 and 51473181), Natural Science Foundation of Zhejiang Province (LQ14E030006) and Natural Science Foundation of Ningbo (2014A610131) are gratefully acknowledged.References
- T. Zhang, H. Yan, L. Shen, Z. Fang, X. Zhang, J. Wang and B. Zhang, RSC Adv., 2014, 4, 48285–48292 RSC.
- Y. H. Guan, W. Liao, Z. Z. Xu, M. J. Chen, J. Q. Huang and Y. Z. Wang, RSC Adv., 2015, 5, 59865–59873 RSC.
- A. B. Morgan and J. W. Gilman, Fire Mater., 2013, 37, 259–279 CrossRef CAS.
- A. R. Horrocks, Polym. Degrad. Stab., 2011, 96, 377–392 CrossRef CAS.
- K. S. Lim, S. T. Bee, L. T. Sin, T. T. Tee, C. T. Ratnam, D. Hui and A. R. Rahmat, Composites, Part B, 2016, 84, 155–174 CrossRef CAS.
- M. Doğan, A. Yılmaz and E. Bayramlı, Polym. Degrad. Stab., 2010, 95, 2584–2588 CrossRef.
- Z. Jiang and G. Liu, RSC Adv., 2015, 5, 88445–88455 RSC.
- S. Bourbigot, M. LeBras, S. Duquesne and M. Rochery, Macromol. Mater. Eng., 2004, 289, 499–511 CrossRef CAS.
- T. Mariappan and C. A. Wilkie, Macromol. Chem. Phys., 2012, 213, 1987–1995 CrossRef CAS.
- Y. Shen, W. Gong, B. Zheng, X. Meng and L. Gao, Polym. Degrad. Stab., 2016, 129, 114–124 CrossRef CAS.
- H. Y. Ma, L. F. Tong, Z. B. Xu and Z. P. Fang, Adv. Funct. Mater., 2008, 18, 414–421 CrossRef CAS.
- X. Wang, Y. Sporer, A. Leuteritz, I. Kuehnert, U. Wagenknecht, G. Heinrich and D. Y. Wang, RSC Adv., 2015, 5, 78979–78985 RSC.
- Y. Q. Ma and Y. Y. Pang, Chin. J. Polym. Sci., 2015, 33, 772–782 CrossRef CAS.
- H. Gui, X. H. Zhang, Y. Q. Liu, W. F. Dong, Q. G. Wang, J. M. Gao, Z. H. Song, J. M. Lai and J. L. Qiao, Compos. Sci. Technol., 2007, 67, 974–980 CrossRef CAS.
- G. B. Huang, S. Q. Chen, S. W. Tang and J. R. Gao, Mater. Chem. Phys., 2012, 135, 938–947 CrossRef CAS.
- S. Peeterbroeck, F. Laoutid, J. M. Taulemesse, F. Monteverde, C. J. M. Lopez, J. B. Nagy, M. Alexandre and P. Dubois, Adv. Funct. Mater., 2007, 17, 2787–2791 CrossRef CAS.
- P. A. Song, L. H. Xu, Z. H. Guo, Y. Zhang and Z. P. Fang, J. Mater. Chem., 2008, 18, 5083–5091 RSC.
- S. P. Nalawade, F. Picchioni and L. P. B. M. Janssen, Prog. Polym. Sci., 2006, 31, 19–43 CrossRef CAS.
- M. Sauceau, J. Fages, A. Common, C. Nikitine and E. Rodier, Prog. Polym. Sci., 2011, 36, 749–766 CrossRef CAS.
- S. D. Yeo and E. Kiran, J. Supercrit. Fluids, 2005, 34, 287–308 CrossRef CAS.
- O. R. Davies, A. L. Lewis, M. J. Whitaker, H. Tai, K. M. Shakesheff and S. M. Howdle, Adv. Drug Delivery Rev., 2008, 60, 373–387 CrossRef CAS PubMed.
- D. L. Tomasko, X. M. Han, D. H. Liu and W. H. Gao, Curr. Opin. Solid State Mater. Sci., 2003, 7, 407–412 CrossRef CAS.
- S. Horsch, G. Serhatkulu, E. Gulari and R. M. Kannan, Polymer, 2006, 47, 7485–7496 CrossRef CAS.
- J. Ma, E. Bilotti, T. Peijs and J. A. Darr, Eur. Polym. J., 2007, 43, 4931–4939 CrossRef CAS.
- D. M. Wu, Q. Y. Meng, Y. Liu, Y. M. Ding, W. H. Chen, H. Xu and D. Y. Ren, J. Polym. Sci., Part B: Polym. Phys., 2003, 41, 1051–1058 CrossRef CAS.
- K. Goren, O. B. Okan, L. Chen, L. S. Schadler and R. Ozisik, J. Supercrit. Fluids, 2012, 67, 108–113 CrossRef CAS.
- K. Wang, S. S. Wang, F. Wu, Y. Y. Pang, W. T. Zhai and W. G. Zheng, Polym. Bull., 2016, 73, 941–957 CrossRef CAS.
- K. Wang, Y. Y. Pang, P. K. Huang, L. H. Zhang, W. Liu and W. G. Zheng, RSC Adv., 2016, 6, 106347–106354 RSC.
- K. Wang, S. S. Wang, F. Wu, Y. Y. Pang, W. Liu, W. T. Zhai and W. G. Zheng, J. Mater. Sci., 2016, 51, 2705–2715 CrossRef CAS.
- K. Wang, Y. Y. Pang, W. Liu, F. Wu and W. G. Zheng, J. Supercrit. Fluids, 2016, 118, 203–209 CrossRef CAS.
- K. Wang, Y. Y. Pang, F. Wu, W. T. Zhai and W. G. Zheng, J. Supercrit. Fluids, 2016, 110, 65–74 CrossRef CAS.
- S. S. Wang, K. Wang, Y. Y. Pang, Y. Li, F. Wu, S. Wang and W. G. Zheng, J. Appl. Polym. Sci., 2016, 133, 43812 Search PubMed.
- M. Lee, C. B. Park and C. Tzoganakis, Polym. Eng. Sci., 1999, 39, 99–109 Search PubMed.
- G. Li, J. Wang, C. B. Park and R. Simha, J. Polym. Sci., Part B: Polym. Phys., 2007, 45, 2497–2508 CrossRef CAS.
- C. B. Park and N. P. Suh, Polym. Eng. Sci., 1996, 36, 34–48 CAS.
- S. Areerat, E. Funami, Y. Hayata, D. Nakagawa and M. Ohshima, Polym. Eng. Sci., 2004, 44, 1915–1924 CAS.
- F. Gao, L. F. Tong and Z. P. Fang, Polym. Degrad. Stab., 2006, 91, 1295–1299 CrossRef CAS.
- L. G. Imhof and K. C. Stueben, Polym. Eng. Sci., 1973, 13, 146–152 CAS.
- M. Zhang, P. Ding and B. J. Qu, Polym. Compos., 2009, 30, 1000–1006 CrossRef CAS.
- Y. W. Yan, L. Chen, R. K. Jian, S. Kong and Y. Z. Wang, Polym. Degrad. Stab., 2012, 97, 1423–1431 CrossRef CAS.
- Y. Li, B. Li, J. Dai, H. Jia and S. Gao, Polym. Degrad. Stab., 2008, 93, 9–16 CrossRef CAS.
- O. G. Sanchez, S. A. Sanchez, F. Calderas, T. L. Medina, V. E. E. Herrera, G. A. Rivera and O. Manero, Polym. Eng. Sci., 2013, 53, 2018–2026 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |