Highly transparent and self-healing films based on the dynamic Schiff base linkage†
Jiaoyu
Ren
a,
Yanxi
Zhu
a,
Hongyun
Xuan
a,
Xuefan
Liu
a,
Zhichao
Lou
bc and
Liqin
Ge
*a
aState Key Laboratory of Bioelectronics, School of Biological Science and Medical Engineering, Southeast University, Nanjing 210096, P. R. China. E-mail: lqge@seu.edu.cn
bCollege of Materials Science and Engineering, Nanjing Forestry University, Nanjing, 210037, P. R. China
cState Key Laboratory of Bioelectronics, Jiangsu Key Laboratory for Biomaterials and Devices, Southeast University, Nanjing, 210096, P. R. China
First published on 2nd December 2016
Abstract
Self-healing optically transparent coatings have attracted significant attention due to their high transparency and ability to heal damages at the same time. In this study, we report an optically transparent and self-healing film via a layer by layer assembly. This film is based on chitosan (CS) and poly(ethylene glycol) functionalized by dialdehyde groups (DF-PEG). The dynamic equilibrium of Schiff base linkage between the aldehyde groups on DF-PEG and amino groups on CS resulted in the self-healing property of the film. The CS/DF-PEG film can heal damages several times at the same location. Moreover, the Schiff base-bonded CS/DF-PEG films are highly transparent with a transmittance of up to 99.47%, and the transmittance of the film can remain at 99% after ten damage/healing processes. The combination of self-healing property and high transparency provides a new method for fabricating the optically transparent devices.
Introduction
Human skin that can heal itself after injury provides an inspiration for designing a new type of functional self-healing materials.1 Recently, worldwide interest has been gained towards the self-healing materials2–4 because self-healing can offer many new and potential applications under various conditions, such as long performance life optical materials. Since Legras et al. found that the thin polymer coatings prepared via layer by layer (LbL) assembly technology have the tendency for self-healing,5 and self-healing films fabricated by LbL technology have attracted considerable attention.1,6–8 When the self-healing film is damaged, an initiator is added to the broken materials to induce self-healing in the materials to rebuild the damaged structures and functions.6,9–11 However, different from the natural self-healing materials that can abidingly repair wounds, most of the artificial functional self-healing films cannot heal wide and deep damage and can only go through a few damage/healing processes.7,12 Thus, the following reasons have been studied by the researchers:8,13,14 (1) amount of raw materials to form the self-healing materials is constant, which limits the ability to heal damages; (2) with an increase in the damage/healing processes in the same region, the distance between the effective healing agents and the broken zone becomes too long for the healing agents to arrive at the broken zone.According to the healing mechanisms, self-healing materials can be divided into two types:1 (1) extrinsic self-healing materials, which complete their self-healing process via releasing healing agents from capsules or other materials encapsulating the healing agent;15 (2) intrinsic self-healing materials, which are based on a dynamic, reversible linkage/reaction, such as hydrogen bonding, reversible acylhydrazone bond formation, Diel–Alder reaction, host–guest interactions, metal–ligand coordination, and supramolecular interactions.3,16–20 Among these, only a few examples21,22 employ the dynamic equilibrium of Schiff base linkages to build the self-healing system. Schiff base-bonded materials can be easily synthesized and used in many fields due to the following reasons: (1) Schiff base-bonded materials provide a new choice for developing antimicrobial materials; (2) catalytic activities of the Schiff base-bonded materials are constant in the presence of water and in high temperature reactions;21 (3) it is easy for the Schiff base-bonded materials to bind with different metal ions. Herein, the first example of Schiff base-bonded film, which was fabricated through the LbL assembly technology, is presented. The combination of self-healing films based on the LbL method and Schiff base-bonded materials will offer many potential applications.
Guided by natural self-healing materials, many self-healing materials with different functionalities, such as antifogging materials, electric materials, and superoleophobic coatings, have been fabricated in recent years.23–25 Self-healing films based on the LbL technology, which can be fabricated on various materials, widen the application range of the self-healing films. Among the various self-healing films based on the LbL technology, films combining the self-healing properties with the optical transparency attract significant attention due to their applications in optical and displaying devices.14 Traces staying in the healed region after damage/healing processes lead to light scattering, which will influence the display of the optical devices. These transparent materials cannot be used in the displaying devices. It is necessary to fabricate a new type of self-healing materials that can restore the original transparency or retain its transparency.
In this study, self-healing (CS/DF-PEG)n films (where n refers to the number of CS layers), which are boned through the Schiff-base linkage between the aldehyde groups on DF-PEG and amino groups on CS, are fabricated via LbL assembly technology. The compositions, microstructure, self-healing ability, and optical transparency of the (CS/DF-PEG)n films have been investigated. The results show that the (CS/DF-PEG)n film combines the self-healing property and high transparency. Moreover, the transparent (CS/DF-PEG)n film can repeatedly heal wide and deep damages in the same location and can retain its transparency at 99% after ten damage/healing processes.
Results and discussion
Characterization of the self-healing films
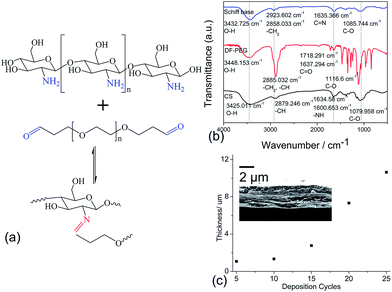
It has been demonstrated that pH has a profound influence on the Schiff base linkage, for instance, Schiff bases are not stable in the acidic range of pH;22 therefore, we deposited CS solution on the glass substrate by spin coating at a speed of 1500 rpm for 20 s instead of immersing the substrate into the CS solution for 10 min. Next, the CS-substrate was immersed in the DF-PEG solution for 10 min to obtain (CS/DF-PEG)1. The Schiff base linkage was formed between the aldehyde groups on DF-PEG and amino groups on CS (Fig. 1(a)). The self-healing film was cross-linked by covalent Schiff base-bond in several seconds. The LbL assembly of CS and DF-PEG blend rapidly produces the deposited (CS/DF-PEG)n coatings (Fig. 1(c)). To obtain the experimental results, (CS/DF-PEG)15 was selected for the following evaluation.The FT-IR spectra of the self-healing film prepared from CS and DF-PEG is presented in Fig. 1(b). To investigate the changes in the chemical bonds, FT-IR spectra of CS and DF-PEG have also been presented. The characteristic peaks of CS appeared at 3425 cm−1 for the hydroxyl group, 2879 cm−1 for the alkyl group, 1635 and 1601 cm−1 for the amide I and II groups, and 1080 cm−1 for the C–O group. In the spectrum of DF-PEG, the peak at 3448 cm−1 was attributed to the O–H stretching vibrations of water molecules and structural OH groups. The adsorption peak at 2885 cm−1 was attributed to the C–H stretching vibration from the alkyl group. The obvious peaks at 1718 cm−1 and 1637 cm−1 were assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration from the aldehyde group. The adsorption peak at 1117 cm−1 was attributed to the C–O stretching vibrations. Compared to CS and DF-PEG, in the spectrum of (CS/DF-PEG)15, many peaks clearly shifted. The O–H peak of the hydroxyl group shifted to 3433 cm−1, whereas peaks representing the C–H stretching vibration from the alkyl group shifted to 2924 cm−1 and 2858 cm−1. A new adsorption peak assigned to the C
O stretching vibration from the aldehyde group. The adsorption peak at 1117 cm−1 was attributed to the C–O stretching vibrations. Compared to CS and DF-PEG, in the spectrum of (CS/DF-PEG)15, many peaks clearly shifted. The O–H peak of the hydroxyl group shifted to 3433 cm−1, whereas peaks representing the C–H stretching vibration from the alkyl group shifted to 2924 cm−1 and 2858 cm−1. A new adsorption peak assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration of the Schiff base linkage appeared at 1635 cm−1 with the disappearance of the C
N stretching vibration of the Schiff base linkage appeared at 1635 cm−1 with the disappearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration from the aldehyde group and N–H stretching vibration from the amide group. The adsorption peaks attributed to the C–O stretching vibrations shifted to 1086 cm−1. This shifting in the peaks proves that CS and DF-PEG can react with one another and form a Schiff base band.
O stretching vibration from the aldehyde group and N–H stretching vibration from the amide group. The adsorption peaks attributed to the C–O stretching vibrations shifted to 1086 cm−1. This shifting in the peaks proves that CS and DF-PEG can react with one another and form a Schiff base band.
Transparency property
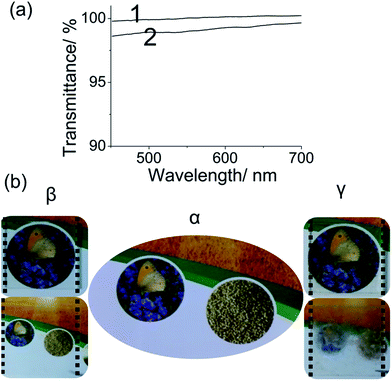
Transmission spectrum, which is obtained by a fibre optic spectrometer, shows that the prepared film is transparent in the visible region, with an average transmittance of 99.47% (Fig. 2(a), curve 2) when the transmittance of glass is 100% (Fig. 2(a), curve 1). Images of a picture with (CS/DF-PEG)15 film and normal self-healing film were obtained to assess the transparency of the prepared films. As shown in Fig. 2(b), an image of the picture can be clearly seen near both (CS/DF-PEG)15 film and normal self-healing film. However, for normal self-healing film, despite its transparency, normal self-healing film covered glass obscures the view of picture, which is placed at a distance of 50 cm behind the film (Fig. 2(b), γ). By contrast, the same picture near the prepared film and picture behind the glass covered with (CS/DF-PEG)15 film can be clearly seen with no difference in their images (Fig. 2(b), β).Self-healing property
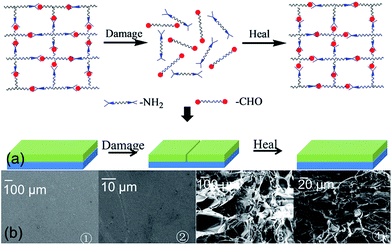
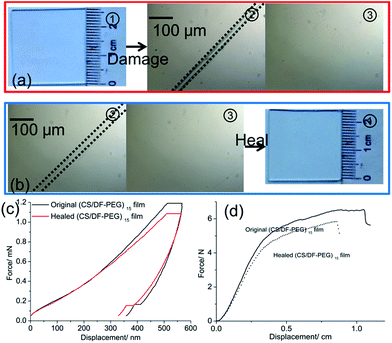
When the (CS/DF-PEG)n film is fabricated, the Schiff base reaction leads to a self-crosslinked network in the film. When the film was damaged, water is added to the broken zone. As shown in Fig. 3(b), the dried film is smooth and compact (Fig. 3(b), ① and ②). Comparing with the dried film, the hydrated state of the self-healing film has a well-defined and interconnected 3D porous network, as imaged for its freeze-dried sample (Fig. 3(b), ③ and ④). The pore sizes of the network are in the range of tens of micrometers to hundreds of micrometers. The results demonstrate that water can diffuse and infiltrate into the film and swell the prepared film, which brings the broken surfaces in contact with each other. Then, the ruptured Schiff base linkage will be reconstructed at this place, and this reconstruction of the Schiff base results in the self-healing of the (CS/DF-PEG)n film (Fig. 3(a)).The dynamic self-healing process of the (CS/DF-PEG)15 film is observed by metallographic microscope. First, a cut of ∼30 μm wide that penetrates to the underlying glass substrate is made on the original (CS/DF-PEG)15 by a scalpel (Fig. 4(a), ②). To bring the broken surfaces in contact with each other, a small amount of ultrapure water is injected into the broken film via a straw. The broken parts expand and come in contact with each other in 20 min. The optical microscope image indicates that the broken parts of the original (CS/DF-PEG)15 film can be totally healed (Fig. 4(a), ③). To understand the healing mechanism of the (CS/DF-PEG)15 film, mechanical properties of an original film and a healed film undergoing one damage/healing process were determined by nanoindentation (Fig. 4(c)). To avoid the influence of the film surface and the underlying substrate, depth of indentation was 500 nm, which is less than one-tenth of the thickness of the film. Hardness is averaged to be 0.175 and 0.171 GPa for the original film and healed film undergoing one damage/healing process, respectively. In the same principle, the Young's moduli of the original film and healed film undergoing one damage/healing process were determined to be 3.46 and 2.79 GPa, respectively. A significant recovery of the hardness of ca. 98% and Young's moduli of ca. 81% is achieved after healing for 20 min. Moreover, a force–displacement curve is used to test the self-healing property of the (CS/DF-PEG)15 film (Fig. 4(d)). When healed for 20 min, compared to the original sample, the breaking displacement and maximal force of the healed film undergoing one damage/healing process were recovered, ca. 81.7% and ca. 89.4%, respectively.
As shown in Fig. 4(a), (CS/DF-PEG)15 film can heal cuts that are tens of micrometres wide with no change in the surface morphology of the prepared film. In the subsequent experiment, the (CS/DF-PEG)15 film is subjected to ten cycles of damage/healing processes and their respective transmittance is monitored by transmission spectroscopy. The (CS/DF-PEG)15 film is damaged with a cut and then healed in deionized water, and the damage/healing process is repeated 10 times (Fig. S1†). According to the results, (CS/DF-PEG)15 film can heal a cut of ∼200 μm wide that penetrates to the underlying glass substrate within 20 min (Fig. S1†). The metallographic microscope images in Fig. 4(b) show that the cuts in the (CS/DF-PEG)15 film are faultlessly healed even after 10 cycles of damage/healing process. Wrinkles caused by the repeating expansion/shrinkage processes are found on the periphery of the healed (CS/DF-PEG)15 film, which have a few influences on the morphology of the prepared film (Fig. 4(a and b), ①). As shown in Fig. 5, the (CS/DF-PEG)15 film can heal wide and deep cuts not only to maintain the structure of the film same, but also to retain its transparency. The original (CS/DF-PEG)15 film is highly transparent, with an average transmittance of ∼99.47%. The atomic force microscopy (AFM) images show that the healed (CS/DF-PEG)15 film undergoing ten damage/healing processes has ∼15 to ∼25 nm grooves and its root-mean-square (RMS) roughness is ∼24.4 nm with an area of 1 × 1 μm, whereas the RMS roughness of the original (CS/DF-PEG)15 film is ∼4.8 nm (Fig. S3†). Grooves that form on the periphery of the healed film after the damage/healing processes cause negligible light scattering. As a result, the healed (CS/DF-PEG)15 film retains its transparency with tiny loss after healing wide and deep cut several times. The transmittance of the healed (CS/DF-PEG)15 film remains at 99% after ten damage/healing processes. To visually observe the transparency of the healed (CS/DF-PEG)15 film, images of a picture placed 50 cm behind the healed (CS/DF-PEG)15 film were obtained (Fig. S2†). As shown in Fig. 5, there is no significant difference in the picture obtained behind the (CS/DF-PEG)15 film, healed five times and ten times, compared with the picture obtained behind the prepared (CS/DF-PEG)15 film.
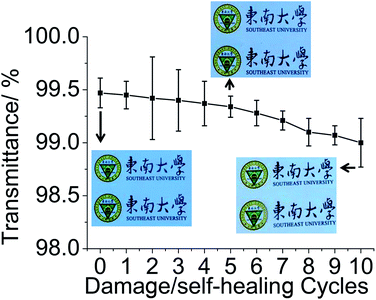 | ||
| Fig. 5 Transmittance of the (CS/DF-PEG)15 film healed for different times; inset shows the pictures behind the healed (CS/DF-PEG)15 film. | ||
Experimental
Materials
Ethanol and glacial acetic acid were bought from Sinopharm Chemical Reagent Shanghai Co. Ltd. CS (Mw = 375![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, the degree of N-deacetylation is 85%) was obtained from Sigma-Aldrich Co. Ltd. DF-PEG (Mw = 3200) was obtained from Laysan Bio, Inc. All other reagents were used without further purification. The glass substrates were soaked in a mixture of 98% H2SO4/30% H2O2 (volumetric ratio 3
000, the degree of N-deacetylation is 85%) was obtained from Sigma-Aldrich Co. Ltd. DF-PEG (Mw = 3200) was obtained from Laysan Bio, Inc. All other reagents were used without further purification. The glass substrates were soaked in a mixture of 98% H2SO4/30% H2O2 (volumetric ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 24 h, rinsed with deionised water, and dried under a N2 stream. A 4 mg mL−1 chitosan solution was prepared by dissolving 40 mg chitosan in 10 mL 1% (w/w) acetic acid aqueous solution. A 3 mg mL−1 DF-PEG solution was obtained by dissolving 15 mg DF-PEG in 4 mL deionized water. These two aqueous polyelectrolyte solutions were used to prepare the self-healing films.
1) for 24 h, rinsed with deionised water, and dried under a N2 stream. A 4 mg mL−1 chitosan solution was prepared by dissolving 40 mg chitosan in 10 mL 1% (w/w) acetic acid aqueous solution. A 3 mg mL−1 DF-PEG solution was obtained by dissolving 15 mg DF-PEG in 4 mL deionized water. These two aqueous polyelectrolyte solutions were used to prepare the self-healing films.
Fabrication of the self-healing films
Preparation of the self-healing films: first, 4 mg mL−1 CS solution was deposited on the treated glass substrate by spin coating at a speed of 1500 rpm for 20 s. Next, to obtain (CS/DF-PEG)1, the CS-substrate was immersed into a 3 mg mL−1 DF-PEG solution for 10 min, followed by rinsing in three water baths for 2 min each to remove the DF-PEG that didn't adsorb on the CS-substrate. The deposition of CS and DF-PEG was repeated 14 times to obtain (CS/DF-PEG)15.Characterization
FT-IR spectra of the prepared samples were obtained using a Nicolet 5700 (Thermo Electron Scientific Instruments Corp.). Scanning electron microscopy (SEM) images were obtained using a field emission scanning electron microscopy (FESEM, Ultra Plus Zeiss). Images were captured using a HUAWEI mobile phone (Honor 7). Optical images and the self-healing process of the samples were observed by metallographic microscope (Olympus BX-51). Transmitted spectrum of the films was obtained from 300 nm to 1000 nm using a fibre optic spectrometer (Ocean Optics, QE65000).Conclusions
In summary, we have demonstrated the fabrication of (CS/DF-PEG)n film with high transparency and self-healing properties based on a LbL technique. The dynamic linkage in Schiff base-bonded (CS/DF-PEG)n film, in which amino groups of CS form Schiff base bonds with the aldehyde groups of DF-PEG endow the film with self-healing property. The transparent (CS/DF-PEG)n film, whose average transmittance is ∼99.47%, can heal ∼200 μm wide damages, which penetrate to the underlying glass substrate, by injecting ultrapure water into the broken film. Moreover, the (CS/DF-PEG)n film can heal such damages several times in the same location. The (CS/DF-PEG)n film can heal wide and deep cuts not only to maintain structure of the film same, but also to retain its transparency. The transmittance of the healed film can remain at 99% after ten damage/healing processes. The combination of self-healing properties and high transparency provides not only a new way to design optically transparent films with self-healing ability to extend service life, but also a new method to solve the problem that the functional materials can only go through a few damage/healing processes. Therefore, the optically transparent and self-healing (CS/DF-PEG)n film can offer potential applications for various devices, such as optical functional device.Acknowledgements
The authors thank for the financial support from the pre-research Foundation for National Science Foundation and the Fundamental Research Funds for the Central Universities. Y. Zhu thanks for the financial support from the Scientific Research Foundation of Graduate School of Southeast University (YBJJ1524), Scientific Innovation Research of College Graduate in Jiangsu Province (KYLX15_0168), Analysis and Testing of the Large-scale Apparatus from the Southeast University and Chinese National Science and Technology support program (2015BAD16B05-5). Z. Lou thanks for the financial support from National Natural Science Foundation of China (61601227) and Nature Science Foundation of Jiangsu Province (BK20160939). Prof. Dr Ge is thankful to Prof. Zhongze Gu's group for their support.Notes and references
- Y. Zhu, H. Xuan, J. Ren and L. Ge, Soft Matter, 2015, 11, 8452–8459 RSC.
- S. Lu, C. Gao, X. Xu, X. Bai, H. Duan, N. Gao, C. Feng, Y. Xiong and M. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 13029–13037 CAS.
- F. Yu, X. Cao, J. Du, G. Wang and X. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 24023–24031 CAS.
- F. Fan, C. Zhou, X. Wang and J. Szpunar, ACS Appl. Mater. Interfaces, 2015, 7, 27271–27278 CAS.
- P. Bertrand, A. Jonas, A. Laschewsky and R. Legras, Macromol. Rapid Commun., 2000, 21, 319–348 CrossRef CAS.
- S. Chen, X. Li, Y. Li and J. Sun, ACS Nano, 2015, 9, 4070–4076 CrossRef CAS PubMed.
- X. Wang, Y. Wang, S. Bi, Y. Wang, X. Chen, L. Qiu and J. Sun, Adv. Funct. Mater., 2014, 24, 403–411 CrossRef CAS.
- X. Wang, F. Liu, X. Zheng and J. Sun, Angew. Chem., Int. Ed., 2011, 50, 11378–11381 CrossRef CAS PubMed.
- N. Pramanik, G. Nando and N. Singha, Polymer, 2015, 69, 349–356 CrossRef CAS.
- G. Li, X. Wu and D. Lee, Sens. Actuators, B, 2015, 221, 1114–1119 CrossRef CAS.
- L. Seéon, P. Lavalle, P. Schaaf and F. Boulmedais, Langmuir, 2015, 31, 12856–12872 CrossRef PubMed.
- M. Barthel, T. Rudolph, A. Teichler, R. Paulus, J. Vitz, S. Hoeppener, M. Hager, F. Schacher and U. Schubert, Adv. Funct. Mater., 2013, 23, 4921–4932 CrossRef CAS.
- M. Caruso, B. Blaiszik, S. White, N. Sottos and J. Moore, Adv. Funct. Mater., 2008, 18, 1898–1904 CrossRef CAS.
- Y. Wang, T. Li, S. Li, R. Guo and J. Sun, ACS Appl. Mater. Interfaces, 2015, 7, 13597–13603 CAS.
- D. Sun, J. An, G. Wu and J. Yang, J. Mater. Chem. A, 2015, 3, 4435–4444 CAS.
- X. Li, Y. Gao, C. Boott, M. Winnik and I. Manners, Nat. Commun., 2015, 6, 8127 CrossRef PubMed.
- J. Syrett, C. Becer and D. Haddleton, Polym. Chem., 2010, 1, 978–987 RSC.
- M. Zhang, D. Xu, X. Yan, J. Chen, S. Dong, B. Zheng and F. Huang, Angew. Chem., Int. Ed., 2012, 51, 7011–7015 CrossRef CAS PubMed.
- T. Cook, Y. Zheng and P. Stang, Chem. Rev., 2013, 113, 734–777 CrossRef CAS PubMed.
- S. Chen, N. Mahmood, M. Beiner and W. Binder, Angew. Chem., Int. Ed., 2015, 54, 10188–10192 CrossRef CAS PubMed.
- K. Gupta, A. Sutar and C. Lin, Coord. Chem. Rev., 2009, 253, 1926–1946 CrossRef CAS.
- F. Ding, S. Wu, S. Wang, Y. Xiong, Y. Li, B. Li, H. Deng, Y. Du, L. Xiao and X. Shi, Soft Matter, 2015, 11, 3971–3976 RSC.
- Y. Wang, T. Li, S. Li and J. Sun, Chem. Mater., 2015, 27, 8058–8065 CrossRef CAS.
- Y. Huang, Y. Huang, M. Zhu, W. Meng, Z. Pei, C. Liu, H. Hu and C. Zhi, ACS Nano, 2015, 9, 6242–6251 CrossRef CAS PubMed.
- K. Chen, S. Zhou and L. Wu, ACS Nano, 2016, 10, 1386–1394 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra23886f |
| This journal is © The Royal Society of Chemistry 2016 |