Biosorption and bioaccumulation of chromate from aqueous solution by a newly isolated Bacillus mycoides strain 200AsB1†
Shan-Shan Wangab,
Shu-Lin Yea,
Yong-He Han *abc,
Xiao-Xia Shic,
Deng-Long Chenbd and
Min Li*a
*abc,
Xiao-Xia Shic,
Deng-Long Chenbd and
Min Li*a
aSchool of Life Sciences, Fujian Normal University, Fuzhou, 350108, China. E-mail: dg1325008@smail.nju.edu.cn; yhhan0303@163.com; mli@fjnu.edu.cn; Fax: +86 591 228 682 05; Tel: +86 591 228 682 05
bQuangang Petrochemical Research Institute, Fujian Normal University, Quanzhou, 362801, China
cState Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Jiangsu 210023, China
dCollege of Environmental Science and Engineering, Fujian Normal University, Fuzhou, 350108, China
First published on 17th October 2016
Abstract
Microbial chromate (Cr6+) reduction and consequent chromite (Cr3+) biosorption have exhibited potential for the remediation of chromium (Cr)-contaminated water. However, few microorganisms that can accumulate amounts of Cr6+ in their cells have been reported. In this study, a new Cr6+-resistant bacterium was isolated and characterized by physiological, biochemical and molecular tests. Its Cr6+ resistant ability, removal efficiency and mechanisms were also investigated at different initial Cr6+ concentrations, solution pHs and incubation temperatures. Results showed that the strain 200AsB1 was a typical bacterium belonging to Bacillus mycoides and tolerated 125 mg L−1 Cr6+ (34 h-LC50 = 63.9 mg L−1). Although the strain preferred to grow at low pH without Cr6+ stress, it only grew well and removed Cr6+ from neutral or alkaline solutions. Similar to the pH experiment, the strain grew better at low temperature than at high temperature, regardless of Cr6+ amendment, but high temperature promoted Cr6+ removal. By analyzing the functional groups' change on the bacterial surface by Fourier transform infrared spectroscopy, our data indicated that N–H and O–H groups from N-methyl-glucamine were involved in Cr6+ adsorption. While Cr concentrations on the bacterial surface were 0.53–0.96 mg L−1, the cell Cr concentration was up to 273 mg kg−1, both of which might contribute to the efficient Cr6+ removal from aqueous solution. Our study demonstrated that B. mycoides strain 200AsB1 could provide new opportunities to remediate Cr-contaminated water through both biosorption and bioaccumulation processes.
1. Introduction
With its good properties such as stiffness and corrosion resistance, chromium (Cr) and its compounds are widely used in industrial applications, including leather tanning, wood preservation, electroplating, alloy formation, and material dyeing and finishing.1,2 However, these activities have led to serious water and soil pollution, making it a high public health concern.3 Chromium often exists in the environment in two oxidation states as chromate (Cr6+, HCrO4− or CrO42−) and chromite (Cr3+); the former is more water-soluble and mobile than the latter.4 While Cr6+ has been classified as a Group A carcinogen due to its mutagenic, teratogenic and carcinogenic nature, Cr3+ is a necessary nutrient for human health.1,3 The maximum permissible level of total Cr in drinking water is 2 mg L−1, but it is often at 50 μg L−1 for Cr6+ and has been regulated to 10 μg L−1 in some regions, e.g., California.1,3 As such, the remediation of Cr-contaminated water and soil depends on the direct removal of Cr6+ by physicochemical methods or indirect treatment by bacterial reduction of Cr6+ to Cr3+.5,6Several methods, such as electrochemical process, ion exchange, liquid extraction, reverse osmosis, membrane process, chemical precipitation, electro coagulation and evaporation, have been developed to remove Cr6+ from wastewater.1 However, these techniques have significant disadvantages, including high costs, high consumption of reagents and energy, non-selective, generation of secondary pollutants and pH dependence.1,6 More and more studies have focused on Cr6+ removal from aqueous solution using biosorbent-based adsorption, of which the most widely used biosorbents are plant and microorganism biomass.7 The adsorption of Cr6+ onto plant or microbial biomass surface is often achieved by van der Waals attraction, covalent binding, ion exchange and complexation.8,9 Plant materials including Ficus auriculata,8 Sterculia guttata,10 Swietenia mahagoni,11 wheat straw,12 Pinus densiflora,13 Caryota urens,14 rice husk,15 Colocasia esculenta16 and Strychnos nux-vomica17 have been used as biosorbents for the removal of Cr6+ from aqueous solution. Microorganisms capable of Cr6+ adsorption include Aeromonas spp., Aspergillus spp., Bacillus spp., Bosea spp., Micrococcus spp., Pseudomonas spp. and Rhizopus spp.2,9,18–22 Unlike surface adsorption, microbial reduction of Cr6+ to Cr3+ is enzymatically catalyzed and exhibits more efficient Cr detoxification.5,9 However, Cr contamination in aqueous solution cannot be completely ended by microorganism-based Cr6+ reduction because the further removal of reduced Cr3+ by Cr6+-reducing microorganisms is limited and Cr3+ is easy to re-oxidize. Studies have shown that some microorganisms can not only adsorb Cr6+ or Cr3+ onto the biomass surface, they also can accumulate them inside cells via sequestration, precipitation and chemical complexation.9,23 It seems that microorganism-based bioaccumulation will be a stable and reliable system to remove Cr6+ from aqueous solution.
In this study, a newly Cr6+-resistant bacterium named Bacillus mycoides 200AsB1 was isolated from agricultural soil. The strain was incubated with Cr6+ at different initial concentrations, initial solution pHs and incubation temperatures. Scanning electron microscopy (SEM) and dose–response fitting were used to investigate its Cr6+ resistant ability and Cr6+ removal efficiency. By determining the Cr concentrations in the washing buffer and digested solutions of bacterial biomass, followed by characterizing by Fourier transform infrared (FT-IR) spectroscopy, the contributions of bioadsorption and bioaccumulation to Cr6+ removal by the strain 200AsB1 were also evaluated.
2. Materials and methods
2.1. Bacteria isolation and culture medium
Soil spiked with 200 mg kg−1 arsenate (As5+) was collected from the rhizosphere of arsenic-hyperaccumulator Pteris vittata. Before use, the soil was rinsed 3 times with sterile Milli-Q water followed by dilution with 0.9% NaCl, and was then streaked onto Luria–Bertani (LB) agar medium (1.5%). After 24 h of growth at 30 °C, 5 colonies with distinct morphology were picked out and purified repeatedly 3 times. Among the 5 isolates, a strain that tolerated 100 mg kg−1 Cr6+ (K2Cr2O7) was selected for further study.The medium used for bacteria isolation and batch experiments contained 5 g L−1 yeast extract, 10 g L−1 peptone, 10 g L−1 NaCl and 1 L Milli-Q water at pH 7.0 ± 0.2.
2.2. Identification of the strain 200AsB1
The physiological and biochemical characteristics of the strain 200AsB1 were tested by Sangon Biotech. Co., Ltd. (Shanghai, China), while the bacteria morphology was characterized by SEM analysis. For molecular identification, the strain was incubated in LB medium for 12 h at 30 °C, 180 rpm and then used for total DNA extraction. Genomic DNA was extracted by using a FastDNA® SPIN Kit for Soil (MP Biomedicals, USA) according to the manufacturer's instructions. The universal bacterial primers (27F: AGAGTTTGATCCTGGCTCAG and 1492R: ACGGCTACCTTGTTACGACTT) were used to amplify 16S rRNA gene by polymerase chain reaction (PCR) performed on a T100 Thermocycler (BioRad, USA). The PCR mixtures contained 25 μL 2× Mix (TransGen Biotech., Beijing, China), 1.5 μL of each 10 μM primer pair, 20 μL of PCR degrade water and 2 μL of DNA template. The PCR programs for amplification of 16S rRNA consisted of pre-denaturation for 5 min at 94 °C, followed by 35 cycles of denaturation and annealing (35 s at 94 °C, 30 s at 55 °C and 1.5 min at 72 °C), and a final extension at 72 °C for 10 min.24 The PCR product was purified and sequenced by GenScript Co., Ltd. (Nanjing, China). The sequence was analyzed by BLAST similarity search against known sequences in the NCBI database. The phylogenetic tree was constructed by using the neighbor-joining algorithm in the MEGA 4.0 program.2.3. Batch experiments for Cr6+ resistance and removal ability identification
To test the impacts of different concentrations of Cr6+ on bacterial growth, before use, the strain was incubated in LB medium for 12 h at 30 °C, 180 rpm. After that, the bacterial suspension was diluted to an optical density (OD600 nm) of 0.321, then aliquots of diluted suspension (2% inoculation) were added to a new LB medium containing 0, 25, 50, 75, 100 and 125 mg L−1 Cr6+. The sampling was conducted every 3 h within a total incubation time of 34 h. OD600 was recorded to evaluate bacterial growth and the supernatant was collected and used to determine the residual Cr6+ and total Cr in the medium. To investigate the Cr6+ removal ability in different treatments, the relative removal content of Cr6+ (mg OD600−1) was also calculated. To evaluate the toxic effects of Cr6+ on bacterial growth, the relationships between Cr6+ concentrations and OD600 were fitted in Origin 9.0 using a nonlinear fitting model DoseResp. Similar to Cr6+ concentration, the effects of medium pH (5.0–9.0) and temperature (20–40 °C) on bacterial growth and Cr6+ removal efficiency were also studied.To have an in situ observation of bacteria morphology under Cr6+ stress, the biomass was collected by centrifugation and pretreated as follows:25 immobilized by 2.5% glutaraldehyde (prepared by PBS) for 4 h, washed three times by PBS (19 mL 0.2 M NaH2PO4 + 81 mL 0.2 M Na2HPO4, pH 7.4), dehydrated by ethyl alcohol at concentrations of 30%, 50%, 70%, 85%, 90% (15 min for each rinse) and 100% (twice, 15 min per rinse), isoamyl acetate (twice, 20 min per rinse) and freeze-dried (FreeZone 6 plus, Labconco, USA) for 48 h. SEM was used to observe cell morphology of the bacteria incubated for 48 h at 30 °C and 180 rpm in the medium spiked with different concentrations of Cr6+ (0, 5, 10, 25, 50 and 75 mg L−1).
2.4. Analysis of Cr adsorption and accumulation by the strain 200AsB1
To determine the potential mechanisms involved in Cr6+ removal from aqueous solution, both Cr6+ biosorption and bioaccumulation by the strain 200AsB1 were tested. Four groups (5, 10, 25 and 50 mg L−1) were selected to investigate the roles of biosorption and bioaccumulation in Cr6+ removal from aqueous solution. In brief, 0.5 g of the washed biomass was digested by using USEPA Method 3050B on a hot block at 105 °C for 8 h (Environmental Express, USA). The washing buffer for triple rinses was also collected. After being centrifuged at 8000 × g for 5 min, the supernatants and washing buffer were diluted using Milli-Q water as required and stored at 4 °C for further determination.Two groups (0 and 50 mg L−1) without washing by PBS were chosen in pairs to further investigate the roles of biosorption in Cr6+ removal by using FT-IR spectroscopy (Thermo Scientific Nicolet iS5, USA). Before determination, the bacterial biomass was mixed with potassium bromide (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w) by grinding on an agate mortar, then molded to a 2 mm slice. Each slice was subjected to FT-IR spectroscopy for full scanning at wavenumbers of 400 to 4000 cm−1.
2, w/w) by grinding on an agate mortar, then molded to a 2 mm slice. Each slice was subjected to FT-IR spectroscopy for full scanning at wavenumbers of 400 to 4000 cm−1.
2.5. Determination of total Cr and Cr6+
The samples were centrifuged at 8000 × g for 5 min, and the supernatant was collected and diluted as required for further use. The concentration of Cr6+ was analyzed by diphenylcarbazide (DPC) colorimetric method,26 while total Cr concentration was determined by the same method after oxidizing Cr3+ to Cr6+ by potassium permanganate (KMnO4). Specifically, the samples were diluted to 20 mL in a 100 mL Erlenmeyer flask and mixed with 0.5 mL (1 + 1) of H2SO4 and 0.5 mL (1 + 1) of H3PO4. After that, required volumes of 4% KMnO4 (w/w) were added into the mixtures to generate a pink color. The samples were then mixed with 1 mL of urea (20%, w/w) and required volumes of 2% NaNO2 (w/w) to make the pink color fade. Once the bubbles disappeared, the samples were transferred to colorimetric tubes and diluted to a total volume of 50 mL. The colorimetric data was determined by using an ultraviolet and visible spectrophotometer (SP-723PC, Shanghai Spectrum Instruments Co., Ltd., China) at 540 nm.2.6. Statistical analysis
All the experiments were conducted in triplicate and the data were presented as the mean of triplicates with standard error. Significant differences were determined according to two-way analysis of variance (ANOVA) by Tukey's multiple comparisons test at P ≤ 0.05 using GraphPad Prism (Release 6.0, USA). The DoseResp fitting was performed using Origin program (Release 9.0, USA). Specifically, the 34 h median lethal concentration (34 h-LC50) was calculated according to the four-parameter model, y = A1 + (A2 − A1)/[1 + 10(LOGx0−x)p], where y is the index, x is the Cr6+ concentration, LOGx0 is the center of DoseResp value (i.e., LC50), A1 and A2 are the bottom and top asymptote respectively, and p is the hill slope.3. Results and discussion
3.1. Isolate identification
A strain, named 200AsB1, that tolerated up to 100 mg L−1 Cr6+ was isolated from a rhizosphere soil containing 200 mg kg−1 As5+. The cells of 200AsB1 were rod-shaped without flagellum and linked end to end (Fig. S1A†). The size of each cell was about 2–4 μm long by 0.8–1.0 μm wide (Fig. S1A†). The cells could form a tight aggregation like a snowflake in the liquid culture (Fig. S2†). We also found that the isolate 200AsB1 produced a characteristic spreading filamentary morphology, a repeating and clockwise pattern, when grown on agar medium (Fig. 1A). The above characteristics indicated that the strain might be a number of Bacillus mycoides, whose morphology is well-known and has been reported previously.27,28To further verify the identification, we tested the physiological, biochemical and molecular characteristics of the isolate 200AsB1. As shown in Table 1, the isolate was a gram positive bacterium and grew well at temperatures of 20–40 °C and solution pH of 5–9. While the Voges–Proskauer and Catalase tests were positive, the indoline test was negative (Table 1). Moreover, the isolate could utilize nitrate, citrate, D-glucose, hydrolyzed starch and gelatin, but it couldn't utilize L-arabinose, D-mannose and D-mannitol (Table 1). Based on its cell and colony morphology and by referring to the Taxonomic Outline of the Prokaryotes Bergey's Manual® of Systematic Bacteriology,29 we concluded that the isolate 200AsB1 belongs to B. mycoides. This conclusion was also supported by 16S rRNA sequencing and subsequent sequence alignment (Fig. 1B). The results showed that the 16S rRNA gene sequence (GenBank accession number KU499950) of the isolate 200AsB1 had 100% similarity to B. mycoides, B. pseudomycoides, B. cereus, B. samanii and B. toyonensis (Fig. 1B). Although B. mycoides is assigned to B. cereus group27 and often shares a high similarity of 16S rRNA with B. cereus, B. pseudomycoides and B. toyonensis,30 our data concluded that the isolate 200AsB1 was a typical strain of B. mycoides.
| Tests | Results/valuesa |
|---|---|
| a + means positive and − means negative. | |
| Gram staining | + |
| Voges–Proskauer test | + |
| Catalase test | + |
| Nitrate reduction | + |
| Citrate | + |
| Indoline test | − |
| Hydrolysis of starch | + |
| Hydrolysis of gelatin | + |
| Utilization of D-glucose | + |
| Utilization of L-arabinose | − |
| Utilization of D-mannose | − |
| Utilization of D-mannitol | − |
| Optimal temperature for growth (°C) | 20–40 |
| Optimal pH for growth | 5–9 |
Since B. mycoides is a fast-growing bacterium and, to the best of our knowledge, its characteristics of Cr6+ biosorption and bioaccumulation have yet to be reported, our study mainly focused on its Cr6+ resistance and Cr6+ removal ability as well as ways of removal under various conditions.
3.2. Chromate resistant ability of the strain 200AsB1
It is known that lots of microorganisms can tolerate high Cr6+ stress due to their efficient Cr6+ adsorption or reduction behaviors.5,9 This was supported by our study, which showed that although a high concentration of Cr6+ (≥75 mg L−1) was toxic to bacterial growth, the strain could tolerate 125 mg L−1 Cr6+ (Fig. 2A and S1†). However, no Cr3+ was observed in the medium, indicating that Cr6+ removal by B. mycoides was not associated with Cr6+ reduction. The strain had an OD600 at 7.691 of the control after 34 h of growth, which was higher than Cr6+ groups (0.338–6.779; Fig. 2A). Similar to bacterial growth, Cr6+ removal efficiency decreased with the increase of Cr6+ concentration in the medium (Fig. 2B). While the highest removal efficiency at 100% was found in the medium spiked with 25 mg L −1 Cr6+ after 25 h of incubation, it was only 16% for 125 mg L−1 Cr6+ (Fig. 2B). However, the relative removal content displayed an opposite trend. For example, only 0.37 mg pf Cr6+ OD600−1 was removed in the medium spiked with 25 mg L−1 Cr6+ after 34 h of growth, being the lowest among five groups (Fig. 2C). Although bacteria couldn't grow well under 125 mg L−1 Cr6+ stress, it had the highest relative removal content up to 8.22 mg of Cr6+ OD600−1 (Fig. 2C).Studies have shown that several typical microorganisms have outstanding ability for Cr6+ removal via biosorption (Table 2). Among them, Rhizopus nigricans had the highest removal capacity of Cr6+ (20 mg L−1 h−1), followed by Bacillus megaterium (6.19 mg L−1 h−1) and Aspergillus sp. (5.11 mg L−1 h−1; Table 2).21,23,31 However, these studies were conducted with a low solution pH (2–5), which might enhance Cr6+ reduction in the presence of organic matter and thus the removal efficiency was over-estimated.7 In fact, if the solution pH was neutral or alkaline, the Cr6+ removal efficiency decreased sharply, e.g., only 0.05 mg L−1 h−1 in Aspergillus foetidus19 and 0.32 mg L−1 h−1 in Pseudomonas aeruginosa (Table 2).2 By comparing to the above microorganisms, B. mycoides was more efficient at Cr6+ removal, having a removal capacity of 2.80 and 2.08 mg L−1 h−1 for 50 and 125 mg L−1 Cr6+, respectively (Table 2). Our data indicated that the strain 200AsB1 was efficient at Cr6+ removal from aqueous solution.
| Microorganisms | Initial concentration (mg L−1) | pH | Time (h) | Removal efficiency (%) | Removal capacity (mg L−1 h−1) | Ref. |
|---|---|---|---|---|---|---|
| a Dry biomass.b Chromate removal by Pseudomonas aeruginosa was achieved by both biosorption and enzymatic reduction. | ||||||
| Aspergillus foetidus | 5 (Cr6+) | 7 | 92 | 97 | 0.05 | Prasenjit and Sumathi19 |
| Aspergillus sp. | 100 (Cr6+) | 5 | 18 | 92 | 5.11 | Congeevaram et al.31 |
| Aspergillus oryzae | 240 (Cr3+) | 5 | 36 | 97 | 6.47 | Nasseri et al.20 |
| Bacillus circulans | 100 (Cr6+) | 2.6 | 24 | 99.4a | 4.14 | Srinath et al.23 |
| Bacillus megaterium | 150 (Cr6+) | 2.6 | 24 | 99.1a | 6.19 | Srinath et al.23 |
| Micrococcus sp. | 100 (Cr6+) | 7 | 18 | 90 | 5.00 | Congeevaram et al.31 |
| Pseudomonas aeruginosa | 30 (Cr6+) | 8 | 72 | 76.7b | 0.32 | Chatterjee et al.2 |
| Rhizopus nigricans | 100 (Cr6+) | 2 | 4 | 80 | 20.0 | Bai and Abraham21 |
| Bacillus mycoides | 50 (Cr6+) | 7 | 34 | 100 | 2.80 | Present study |
| 125 (Cr6+) | 7 | 31 | 25 | 2.08 | ||
Although the strain 200AsB1 tolerated high concentrations of Cr6+, Cr6+ stress also showed toxicity to bacterial cells (Fig. S1B–F†). To evaluate bacterial tolerance to Cr6+, we also calculated the 34 h-LC50 based on the DoseResp model. Results showed that the 34 h-LC50 was 63.92 mg L−1 (Fig. 2D). Therefore, the Cr6+ concentration of 50 mg L−1 was selected for further use.
3.3. Low pH promoted bacterial growth and Cr6+ removal
In the environment, bacteria are sensitive to pH change. For example, soil acidification often reduces bacterial communities, which may be associated with the ecological filtering, evolutionary dispersal and mediation of nutrient availability.32,33 It is assumed that bacterial growth in liquid culture is also regulated by solution pH due to the change of membrane charge and nutrient availability.25To test the impact of solution pH on bacterial growth and Cr6+ removal in B. mycoides strain 200AsB1, the medium pH was adjusted to 5, 6, 7, 8 and 9 and the bacteria was incubated for 72 h at 30 °C with the shaking speed at 180 rpm. As shown in Fig. 3A and B, the strain grew well at all pHs ranging from 5.0 to 9.0, but under Cr6+ stress it only grew well in the neutral or alkaline medium. Without Cr6+ amendment, the bacteria had the highest OD600 at 36 h, being up to 10.7 at pH 6.0 (Fig. 3A). It seemed that the strain 200AsB1 preferred to grow in the low pH medium (Fig. 3A). Once spiked with 50 mg L−1 Cr6+, however, the highest OD600 at 36 h decreased to 6.21 at pH 8.0 and no bacterial growth was observed at pH 5–6 (Fig. 3B). Moreover, after 36 h, the bacteria displayed a similar growth trait in both the solutions with or without Cr6+ (Fig. 3A and B). Our data were consistent with previous studies, which showed that B. mycoides often grew well and enzymatically functioned at pH 7–9.34,35
As expected, Cr6+ removal efficiency was consistent with bacterial biomass (OD600), i.e., 50 mg L−1 Cr6+ was completely removed within 36 h when three groups crossed with each other (Fig. 3B and C). It was interesting to note that, however, 33% and 21% of Cr6+ disappeared at 36 h although no bacterial growth was found (Fig. 3B and C). Since acid solution can enhance Cr6+ reduction in the presence of organic matter,7 we tested the total Cr and found that it was ∼30% and 20% higher than the Cr6+ concentration at pH 5 and pH 6, respectively (data not shown). We thus hypothesized that the LB medium with low pH resulted in considerable Cr6+ reduction. To test whether Cr3+ displayed toxicity to bacterium, the strain 200AsB1 was subjected to the medium spiked with 50 or 100 mg L−1 Cr3+ (chromic chloride). However, the strain grew well in all treatments (data not shown). Since Cr3+ often presents as insoluble compounds and is difficult to cross bacterial cell membranes with a low efficiency,36 some unknown change, except for Cr3+ production, inhibited bacterial growth and warranted further investigation.
3.4. Low temperature promoted bacterial growth but high temperature enhanced Cr6+ removal
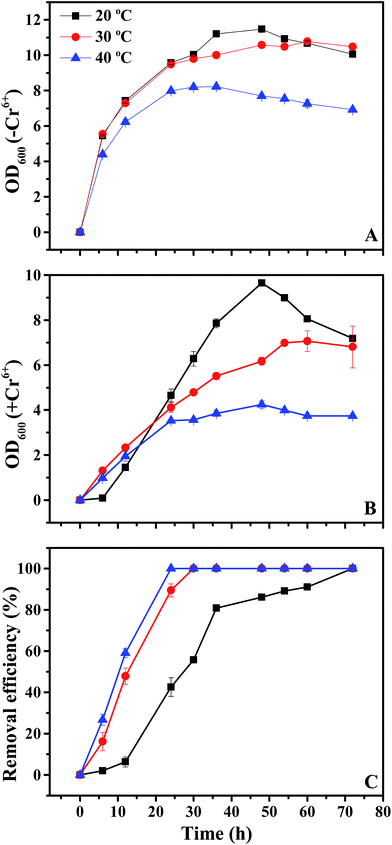
In addition to pH, temperature is another parameter that can impact bacterial growth via regulating substrate sequestration and enzyme activity.37,38 To determine how bacteria responded to temperature, we also tested bacterial growth and Cr6+ removal efficiency at temperatures ranging from 20 °C to 40 °C. In the control without Cr6+ stress, the strain grew best at the lowest temperature of 20 °C (48 h-OD600 = 11.5), followed by 30 °C (48 h-OD600 = 10.6) and 40 °C (48 h-OD600 = 7.69; Fig. 4A). Although Cr6+ amendment decreased bacterial growth by 16–45%, it followed a similar trend to no Cr6+ treatments. For example, the strain had an OD600 at 9.65 at the lowest temperature of 20 °C, which was higher than that at 30 °C (OD600 = 6.17) and 40 °C (OD600 = 4.25; Fig. 4B). This was different from the documented data, which showed that B. mycoides often grows best at 30 ± 0.2 °C.29Unlike bacterial growth, Cr6+ removal efficiency increased with the increase of temperature (Fig. 4B). For example, Cr6+ removal efficiency reached 100% within 24 h at 40 °C, while it took 30 h and 72 h to reach similar results at 30 °C and 20 °C, respectively (Fig. 4B). This indicated that Cr6+ removal by the strain 200AsB1 might be attributed to its biosorption onto the biomass surface because it is often verified as an endothermic process.1,39 Moreover, since CrO42− and sulfate (SO42−) are chemical analogs, Cr6+ accumulation by microorganisms is also likely via the proton–anion symport system that has been proven to transport SO42−.36,40 As such, the active transport of CrO42− by the proton–anion symport system might be enhanced by high temperature, similar to the glycerol/H+ symporter in Saccharomyces cerevisiae41 and the Na+/H+/glutamate transporter in B. stearothermophilus and B. caldotenax.42 Therefore, the low OD600 at high temperature was also likely due to the higher Cr6+ biosorption onto the biomass surface and/or accumulation inside cells, thus showing higher Cr toxicity to bacterial growth (Fig. 4B).
3.5. Both biosorption and bioaccumulation contributed to Cr6+ removal
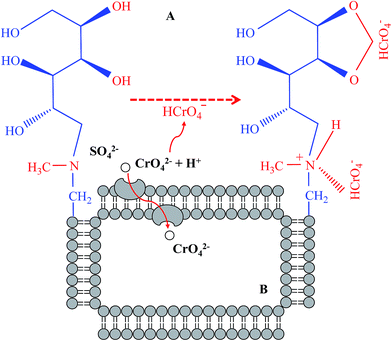
As previously described, Cr6+ removal might be achieved by both biosorption on the biomass surface and bioaccumulation inside cells, which was supported by the fact that Cr6+ removal efficiency increased with bacterial biomass (OD600) (Fig. 2A, B and 3B, C). To further verify this hypothesis, we tested Cr6+ concentrations on the biomass surface and inside bacterial cells.As expected, considerable Cr6+ was observed on the biomass surface, being the highest concentration at 0.96 mg L−1 in the Cr5 group (Fig. 5A). The lowest concentration of Cr6+ was 0.53 mg L−1 in the Cr50 group, which might be in this case due to the high toxicity of Cr6+ to bacteria as OD600 decreased from 10.3 to 7.0 with the increase of Cr6+ from 5 to 50 mg L−1 (Fig. 5A). However, no Cr3+ was detected in all treatments, indicating that Cr6+ reduction didn't happen. To reveal the adsorption mechanisms of Cr6+ onto the biomass surface, the FT-IR spectra of bacterial biomass collected from the medium with or without 50 mg L−1 Cr6+ were also determined (Fig. 5B). As shown in Fig. 5B(a), the broad peak and strong band at 3312 cm−1 is likely to be due to the overlap of O–H and N–H stretching vibrations, indicating the presence of both surface free hydroxyl groups and chemisorbed water.43,44 The peaks at around 2932 cm−1 and 1384 cm−1 are assigned to the C–H symmetric stretch of the methylene groups (–CH2) and deformation vibration of methyl groups (–CH3),43,44 and the latter may also correspond to sulfonamide groups.45 The peaks at 1654 and 1452 cm−1 can be attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of carboxylate (–COO−) or the mode with N–H deformation vibration of amide I groups.43–45 The absorbance at 1539 cm−1 is usually associated with the amide-II groups, which is attributable to N–H bending and C–N stretching in protein amide groups.45 The peaks at 1243 and 1056 cm−1 are due to stretching of C–O in ketones, aldehydes and lactones or carboxyl groups on the biomass surface.44,46 The peaks at 1056 and 800 cm−1 (δC–N) also suggest the presence of N-methyl-glucamine in the biomass surface, while the peak at 552 cm−1 indicates the presence of C–O in hydroxyl groups from N-methyl-glucamine.47 Some shifts in wave numbers from 1384 to 1397 cm−1, 1056 to 1071 cm−1 (δC–O), and 800 to 879 cm−1 were noticed in the spectra of dried B. mycoides before and after use (Fig. 5B). All these changes, especially for the new band at 879 cm−1, suggest that N–H in N-methyl-glucamine contributes to Cr6+ biosorption onto the bacterial surface, which has been verified previously by Gandhi et al.47 and Pan et al.3 Moreover, the peak at 552 cm−1 becomes narrow and shifts to a lower frequency, confirming that Cr6+ sorption has also occurred on the biomass surface.47 The proposed mechanisms of Cr6+ adsorption onto bacterial biomass are shown in Fig. 6A.
O stretching vibration of carboxylate (–COO−) or the mode with N–H deformation vibration of amide I groups.43–45 The absorbance at 1539 cm−1 is usually associated with the amide-II groups, which is attributable to N–H bending and C–N stretching in protein amide groups.45 The peaks at 1243 and 1056 cm−1 are due to stretching of C–O in ketones, aldehydes and lactones or carboxyl groups on the biomass surface.44,46 The peaks at 1056 and 800 cm−1 (δC–N) also suggest the presence of N-methyl-glucamine in the biomass surface, while the peak at 552 cm−1 indicates the presence of C–O in hydroxyl groups from N-methyl-glucamine.47 Some shifts in wave numbers from 1384 to 1397 cm−1, 1056 to 1071 cm−1 (δC–O), and 800 to 879 cm−1 were noticed in the spectra of dried B. mycoides before and after use (Fig. 5B). All these changes, especially for the new band at 879 cm−1, suggest that N–H in N-methyl-glucamine contributes to Cr6+ biosorption onto the bacterial surface, which has been verified previously by Gandhi et al.47 and Pan et al.3 Moreover, the peak at 552 cm−1 becomes narrow and shifts to a lower frequency, confirming that Cr6+ sorption has also occurred on the biomass surface.47 The proposed mechanisms of Cr6+ adsorption onto bacterial biomass are shown in Fig. 6A.
In addition to surface Cr, we also determined the Cr concentration inside bacterial cells. Unlike the Cr6+ concentration on the biomass surface, the Cr concentration inside bacterial cells increased with the medium Cr6+, being highest at 273 mg kg−1 (Fig. 5C). This indicated that higher Cr accumulation inside bacterial cells resulted in higher toxicity to bacterial growth, i.e., while the Cr concentration increased from 20.5 to 273 mg kg−1, bacterial OD600 decreased from 10.3 to 7.0 (Fig. 5A and C). As such, we concluded that B. mycoides strain 200AsB1 could remove Cr6+ from aqueous solution via both bioadsorption and bioaccumulation, and the latter might also be achieved by the SO42− transporting system, as previously described (Fig. 6B).
4. Conclusions
In our study, a new Cr6+-resistant bacterium B. mycoides strain 200AsB1 was isolated from a rhizosphere soil. Results showed that the strain tolerated 125 mg L−1 Cr6+ and displayed high efficiency for Cr6+ removal. While the strain grew better at low pHs, it removed Cr6+ more efficiently at high pHs. Similarly, although the strain grew better in the medium at low temperature than that at high temperature, Cr6+ removal efficiency displayed an opposite result. Our data demonstrated that efficient Cr6+ removal by the strain 200AsB1 might be associated with both the adsorption on the biomass surface by N–H and O–H from N-methyl-glucamine and bioaccumulation inside bacterial cells. Future studies should focus on its applications in the remediation of Cr6+-contaminated water.List of symbols
| y | Dependent variable of the DoseResp fitting model (OD600) |
| x | Independent variable of the DoseResp fitting model (mg L−1) |
| LOGx0 | The center of DoseResp value (i.e., LC50, mg L−1) |
| A1 | Bottom asymptote of the DoseResp fitting model |
| A2 | Top asymptote of the DoseResp fitting model |
| p | The hill slope of the DoseResp fitting model |
| P | LSD test value at α = 0.05 |
| δC–N | δ value of C–N in functional groups (cm−1) |
| δC–O | δ value of C–O in functional groups (cm−1) |
| LC50 | Lethal concentration 50 (mg L−1) |
| OD600 | Optical density at wavenumber of 600 nm |
| w | Weight of the chemicals (g) |
Acknowledgements
We would like to express our thanks for the help from Yu-Xuan Ye at the State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University for the FT-IR analysis. We also thank two anonymous referees, copy-editor and Dr Xin Bai at the Quangang Petrochemical Research Institute, Fujian Normal University for helping with the manuscript improvement and spelling and grammar correction. This work was supported in part by the Science and Technology Program of Fujian Province (2017Y01010165) and the Quanzhou Projects in the Science and Technology Program (2015Z105).References
- M. E. A. Abigail, M. S. Samuel and R. Chidambaram, J. Taiwan Inst. Chem. Eng., 2014, 49, 156–164 CrossRef
.
- S. Chatterjee, I. Ghosh and K. K. Mukherjea, Curr. Sci., 2011, 101, 645–652 CAS
.
- Y. Pan, D.-X. Guan, D. Zhao, J. Luo, H. Zhang, W. Davison and L. Q. Ma, Environ. Sci. Technol., 2015, 49, 14267–14273 CrossRef CAS PubMed
.
- M. Megharaj, S. Avudainayagam and R. Naidu, Curr. Microbiol., 2003, 47, 51–54 CrossRef CAS PubMed
.
- H. Thatoi, S. Das, J. Mishra, B. P. Rath and N. Das, J. Environ. Manage., 2015, 146, 383–399 CrossRef PubMed
.
- C. Rosales-Landeros, C. Barrera-Díaz, B. Bilyeu, V. Guerrero and F. Núñez, Am. J. Anal. Chem., 2013, 4, 8–16 CrossRef
.
- D. Park, Y.-S. Yun and J. M. Park, J. Hazard. Mater., 2006, 137, 1254–1257 CrossRef CAS PubMed
.
- S. Rangabhashiyam, E. Nakkeeran, N. Anu and N. Selvaraju, Res. Chem. Intermed., 2015, 41, 8405–8424 CrossRef CAS
.
- H. Zhang, L. Liu, Q. Chang, H. Wang and K. Yang, Can. J. Microbiol., 2015, 61, 399–408 CrossRef CAS PubMed
.
- S. Rangabhashiyam and N. Selvaraju, J. Mol. Liq., 2015, 207, 39–49 CrossRef CAS
.
- S. Rangabhashiyam and N. Selvaraju, J. Mol. Liq., 2015, 209, 487–497 CrossRef CAS
.
- X. Yao, S. Deng, R. Wu, S. Hong, B. Wang, J. Huang, Y. Wang and G. Yu, RSC Adv., 2016, 8797–8805 RSC
.
- D. Park, Y.-S. Yun, D. S. Lee and J. M. Park, Desalination, 2011, 271, 309–314 CrossRef CAS
.
- E. Suganya, S. Rangabhashiyam, A. V. Lity and N. Selvaraju, Desalin. Water Treat., 2016, 57, 23940–23950 CrossRef CAS
.
- D. Ding, X. Ma, W. Shi, Z. Lei and Z. Zhang, RSC Adv., 2016, 6, 74675–74682 RSC
.
- E. Nakkeeran, N. Saranya, M. S. G. Nandagopal, A. Santhiagu and N. Selvaraju, Int. J. Phytorem., 2016, 18, 812–821 CrossRef CAS PubMed
.
- E. Nakkeeran, S. Rangabhashiyam, M. S. G. Nandagopal and N. Selvaraju, Desalin. Water Treat., 2016, 57, 23951–23964 CrossRef CAS
.
- M. X. Loukidou, T. D. Karapantsios, A. I. Zouboulis and K. A. Matis, Ind. Eng. Chem. Res., 2004, 43, 1748–1755 CrossRef CAS
.
- B. Prasenjit and S. Sumathi, J. Mater. Cycles Waste Manage., 2005, 7, 88–92 CrossRef CAS
.
- S. Nasseri, M. M. Assadi, M. N. Sepehr, K. Rostami, M. Shariat and K. Nadafi, Pak. J. Biol. Sci., 2002, 5, 1056–1059 CrossRef
.
- R. S. Bai and T. E. Abraham, Bioresour. Technol., 2001, 79, 73–81 CrossRef
.
- T. Sathvika, Manasi, V. Rajesh and N. Rajesh, RSC Adv., 2015, 5, 107031–107044 RSC
.
- T. Srinath, T. Verma, P. W. Ramteke and S. K. Garg, Chemosphere, 2002, 48, 427–435 CrossRef CAS PubMed
.
- Y. H. Han, J. W. Fu, P. Xiang, Y. Cao, B. Rathinasabapathi, Y. Chen and L. Q. Ma, J. Hazard. Mater., 2017, 321, 146–153 CrossRef CAS PubMed
.
- Y. Han, W. Zhang, Z. Zhuang, Z. Zhou, X. Xu and M. Li, Acta Microbiol. Sin., 2013, 53, 47–58 CAS
.
- L. S. Clesceri, A. E. Greenberg and A. D. Eaton, Standard Methods for the Examination of Water and Wastewater 20th, American Public Health Association, 1998 Search PubMed
.
- C. D. Franco, E. Beccari, T. Santini, G. Pisaneschi and G. Tecce, BMC Microbiol., 2002, 2 DOI:10.1186/1471-2180-2-33
.
- J. P. Stratford, M. A. Woodley and S. Park, PLoS One, 2013, 8 DOI:10.1371/journal.pone.0081549
.
- G. M. Garrity, J. A. Bell and T. G. Lilburn, Taxonomic outline of the prokaryotes. Bergey's manual® of systematic bacteriology, Springer, Berlin, Germany, 2004 Search PubMed
.
- C. Ash, J. A. E. Farrow, M. Dorsch, E. Stackebrandt and M. D. Collins, Int. J. Syst. Evol. Microbiol., 1991, 41, 343–346 CAS
.
- S. Congeevaram, S. Dhanarani, J. Park, M. Dexilin and K. Thamaraiselvi, J. Hazard. Mater., 2007, 146, 270–277 CrossRef CAS PubMed
.
- X.-M. Zhang, W. Liu, G.-M. Zhang, L. Jiang and X.-G. Han, Soil Biol. Biochem., 2015, 81, 275–281 CrossRef CAS
.
- K. Zhalnina, R. Dias, P. D. de Quadros, A. Davis-Richardson, F. A. Camargo, I. M. Clark, S. P. McGrath, P. R. Hirsch and E. W. Triplett, Microb. Ecol., 2015, 69, 395–406 CrossRef CAS PubMed
.
- M. A. Abdel-Naby, A.-M. S. Ismail, S. A. Ahmed and A. F. A. Fattah, Bioresour. Technol., 1998, 64, 205–210 CrossRef CAS
.
- A. R. Najafi, M. R. Rahimpour, A. H. Jahanmiri, R. Roostaazad, D. Arabian and Z. Ghobadi, Chem. Eng. J., 2010, 163, 188–194 CrossRef CAS
.
- M. I. Ramírez-Díaz, C. Díaz-Pérez, E. Vargas, H. Riveros-Rosas, J. Campos-García and C. Cervantes, BioMetals, 2008, 21, 321–332 CrossRef PubMed
.
- S. L. Herendeen, R. A. VanBogelen and F. C. Neidhardt, J. Bacteriol., 1979, 139, 185–194 CAS
.
- D. B. Nedwell, FEMS Microbiol. Ecol., 1999, 30, 101–111 CrossRef CAS PubMed
.
- M. Danish, R. Hashim, M. N. M. Ibrahim, M. Rafatullah and O. Sulaiman, J. Anal. Appl. Pyrolysis, 2012, 97, 19–28 CrossRef CAS
.
- H. Cypionka, Arch. Microbiol., 1987, 148, 144–149 CrossRef CAS
.
- C. Ferreira and C. Lucas, FEBS Lett., 2007, 581, 1923–1927 CrossRef CAS PubMed
.
- B. Tolner, B. Poolman and W. N. Konings, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1997, 118, 423–428 CrossRef CAS PubMed
.
- C.-Y. Tan, M. Li, Y.-M. Lin, X.-Q. Lu and Z.-L. Chen, Desalination, 2011, 266, 56–62 CrossRef CAS
.
- A. Sarı and M. Tuzen, J. Hazard. Mater., 2008, 157, 448–454 CrossRef PubMed
.
- S. Chakravarty, A. Mohanty, T. N. Sudha, A. K. Upadhyay, J. Konar, J. K. Sircar, A. Madhukar and K. K. Gupta, J. Hazard. Mater., 2010, 173, 502–509 CrossRef CAS PubMed
.
- S. Chandrasekhar and P. N. Pramada, Adsorption, 2006, 12, 27–43 CrossRef CAS
.
- M. R. Gandhi, N. Viswanathan and S. Meenakshi, Ion Exch. Lett., 2010, 3, 25–35 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra23879c |
| This journal is © The Royal Society of Chemistry 2016 |