Efficient sensing of fluoride ions in water using a novel water soluble self-assembled supramolecular sensor based on pillar[5]arene†
Qi Lin*,
Feng Zheng,
Lu Liu,
Peng-Peng Mao,
You-Ming Zhang,
Hong Yao and
Tai-Bao Wei*
Key Laboratory of Eco-Environment-Related Polymer Materials, Ministry of Education of China, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, 730070, P. R. China. E-mail: linqi2004@126.com; weitaibao@126.com
First published on 14th November 2016
Abstract
The development of novel strategies to achieve efficient detection of ions in water is an intriguing challenge. Moreover, due to its high hydration enthalpy, the detection of F− in water is a very difficult task. Herein, we report a novel and efficient method for highly selective detection of ions in water. This novel approach can be illustrated as follows. First, water soluble cationic pillar[5]arene (P5) and aromatic acid derivatives (N1) that were self-assembled in water formed supramolecular sensors (P5N1). Then, competitive coordination was rationally introduced into these supramolecular sensors by adding metal ions (such as Fe3+) to form new supramolecular sensors (P5N1Fe). Interestingly, the supramolecular sensor P5N1Fe showed a high selectivity for F− in water solution. Therefore, this suggests that this method is a novel and facile way to design sensors for the efficient sensing of ions in water.
Introduction
As we all know, ions play a fundamental role in many chemical, biological, medical and technological processes.1–4 Generally, most biological and environmental procedures are carried out in water systems. Therefore, it is very important to detect ions in water solutions. To date, there have been various useful strategies or methods, such as the design of water soluble receptors or using host/guest complexes as water soluble sensors, employed to develop chemosensors that could efficiently work in water.5–8 However, these methods typically suffer from a number of problems. First, owing to the poor water solubility of most organic groups, it is very difficult to simultaneously introduce a signal group and binding sites into a single water soluble chemosensor. Second, host/guest complex chemosensors easily suffer from interference from other guest ions. Finally and more importantly, numerous ions have a high hydration enthalpy, which blocks the binding between ions and sensors. For instance, the hydration enthalpy (ΔH°) of fluoride (F−) is −504 kJ mol−1.9–12 Consequently, the development of novel strategies or approaches to achieve efficient detection of ions in water is still an intriguing challenge.Recently, pillararene chemistry has experienced rapid development13–18 and pillararene derivatives are now widely used in the field of molecular recognition,19–22 drug delivery,23–27 molecular devices28–34 and so on. It's worth mentioning that there are two types of important water soluble pillararenes developed to date: cationic pillararene35 and anionic pillararene.36,37 Due to their good water solubility, inclusion and self-assembly properties, these types of pillararene derivatives provide a great opportunity for the design of water soluble chemosensors.
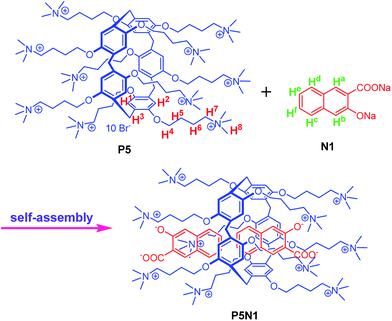
In view of these and based on our research in ion recognition,38–44 by rationally introducing competitive coordination into a self-assembled supramolecular chemosensor, we developed a novel and efficient way to design chemosensors that could work efficiently in water. Our strategy was as follows. First, as shown in Scheme 1, we used water soluble cationic pillar[5]arene (P5) as the host and the aromatic acid derivative N1 as the guest. In N1, the naphthyl groups act as fluorescent signal groups and π–π interaction sites, whereas, the acid groups act as hydrophilic groups and binding sites. Second, the pillararene P5 and aromatic acid N1 could self-assemble into a supramolecular system, which could act as a supramolecular chemosensor P5N1. Finally, in order to achieve high selectivity, we rationally introduced competitive coordination by adding metal ions (such as Fe3+) into the supramolecular system to form a new supramolecular sensor P5N1Fe (Scheme 2). Interestingly, this strategy achieved great success. By the competitive coordination between Fe3+, F− and the aromatic acid N1 in the supramolecular sensors, P5N1 could separately sense Fe3+ in water, whereas P5N1Fe could sense F− in water with high selectivity and sensitivity.
 | ||
| Scheme 2 Self-assembly process of the supramolecular sensor P5N1Fe and the proposed ions sensing mechanism based on competitive coordination. | ||
Results and discussion
The cationic pillar[5]arene (P5) was synthesized according to Scheme S1.† The self-assembly properties of P5 with N1 were investigated via 1H MNR spectroscopy in D2O. As shown in Fig. 1 and S1–S3,† when P5 was mixed with N1, the protons' peaks on N1 and P5, respectively, showed evident shifts. More specifically, the triplet peaks of He and Hf on N1 showed dramatic upfield shifts (Δδ = −1.01 and −0.92 ppm) and became broad. A possible reason for this was that these protons were located in the cavity of P5 and were thus shielded by the electron-rich cylinder. Moreover, the double peaks of Hd and Hc on N1 showed moderate upfield shifts (Δδ = −0.20 and −0.39 ppm), which could be attributed to the slipped-parallel type π–π interactions45 between the naphthyl group of N1 and the P5 electron-rich cylinder. These slipped-parallel type π–π interactions also induced single peaks of H1, H2 and H3 on P5 to carry out moderate upfield shifts (Δδ = −0.17 and −0.23 ppm). Moreover, the single peak of Ha on N1 showed a moderate downfield shift (Δδ = 0.29 ppm), which could be attributed to the electrostatic interaction between the carboxylate group on N1 and the quaternary ammonium group on P5. These electrostatic interactions induced charge transfer from the naphthyl carboxylate group to the quaternary ammonium group on P5. For the same reason, the peaks of H3–8 on P5 showed upfield shifts. According to these results, the proposed self-assembly mechanism is shown in Scheme 1, where the partial naphthyl group of N1 was located in the cavity of P5, and slipped-parallel type π–π interactions exist between the naphthyl group of N1 and the P5 cylinder. Moreover, the carboxylate groups of N1 were bonded to the quaternary ammonium groups on P5 via electrostatic interactions. By this means, P5 and N1 self-assembled into a supramolecular sensor P5N1.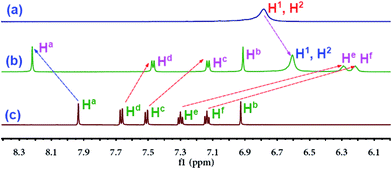 | ||
Fig. 1 Partial 1H NMR of the D2O solution of (a) P5 (1 × 10−3 mol L−1), (b) mixture of P5 (1 × 10−3 mol L−1) and N1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (c) N1 (2 × 10−3 mol L−1). 2) (c) N1 (2 × 10−3 mol L−1). | ||
Mass spectrometry experiments also supported the above proposed self-assembly mechanism. In the ESI-MS of the supramolecular sensor P5N1 (Fig. S5†), a peak at m/z 611.55 for [P5 + 2N1 + 4Br− + 2H+]4+ was monitored, which proved the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexation stoichiometry between P5 and N1. Moreover, the Job plot (Fig. S5†) also supported this result.
2 complexation stoichiometry between P5 and N1. Moreover, the Job plot (Fig. S5†) also supported this result.
Further evidence for the formation of the self-assembled supramolecular sensor P5N1 was received from fluorescent spectroscopy. As shown in Fig. 2, the water solution of N1 showed green fluorescence at λem = 510 nm, whereas the water solution of P5 had no fluorescence. Upon the addition of the P5 solution into the N1 solution, the fluorescence of N1 showed a significant increase. This phenomenon indicated that P5 carried out a self-assembly with N1 and formed the corresponding supramolecular chemosensor P5N1. The association constant for the complexation between P5 and N1 was estimated by means of fluorescence titrations (Fig. S6†) at room temperature in water. The association constant (Ka) for P5N1 was calculated to be 9.94 × 109 M−2.
The stability of the supramolecular sensor P5N1 was also examined over a wide range of pH values in HEPES buffered water solution (Fig. 3). From the results, the supramolecular sensor P5N1 showed good stability in the pH range 5.0–11.0.
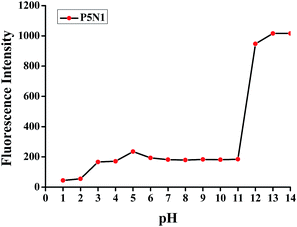 | ||
| Fig. 3 Influence of pH on the fluorescence of P5N1 in HEPES buffered water solution (1 × 10−5 mol L−1). | ||
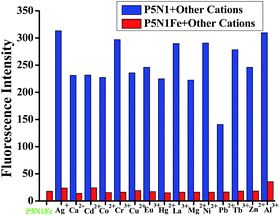
In order to investigate the ions' response property of the supramolecular sensor P5N1, a series of metal ions, including Al3+, Mg2+, Ca2+, Fe3+, Co2+, Ni2+, Cu2+, Ag+, Zn2+, Cd2+, Hg2+, Pb2+, Cr3+, Tb3+, Eu3+ and La3+, were separately added into the water solution of P5N1. Interestingly, the supramolecular sensor P5N1 showed a selectivity response for Fe3+. Indeed, when adding various metal ions into the P5N1 water solution, only Fe3+ could induce the quenching of the fluorescence of P5N1 at λem = 510 nm (Fig. 4 and S7†).
Moreover, the competitive experiments (Fig. 5) indicated that other cations could not induce any significant interference into the sensing process of P5N1 for Fe3+. Therefore, P5N1 could act as a selective Fe3+ sensor. In addition, the supramolecular sensor P5N1 showed high sensitivity for Fe3+. Based on fluorescent titrations (Fig. S8 and S9†), the detection limit of P5N1 for Fe3+ was 8.24 × 10−7 mol L−1.
According to the above results, we rationally introduced competitive coordination into the supramolecular sensor P5N1 via separately adding Fe3+ into the P5N1. The Fe3+ could be coordinated with the carboxylate groups in P5N1 to form the corresponding new supramolecular sensor P5N1Fe.
Interestingly, by competitive coordination, the supramolecular sensor P5N1Fe showed a selective fluorescence response for F− in water solution. As shown in Fig. 6, with the addition of water solutions of various anions (F−, Cl−, Br−, I−, AcO−, H2PO4−, HSO4−, SCN−, CN− and ClO4−) into the supramolecular sensor P5N1Fe, only F− could induce the P5N1Fe water solution to show a dramatic fluorescence increase at λem = 510 nm and emit green fluorescence (Fig. S10†).
Moreover, the competitive experiments (Fig. 7) indicated that other anions could not induce any significant interference into the sensing process of P5N1 for Fe3+ or P5N1Fe for F−. Therefore, P5N1Fe could act as a selective F− sensor. In addition, the supramolecular sensor showed high sensitivity for F−. Based on fluorescence titrations (Fig. S11 and S12†), the detection limit of P5N1Fe for F− was 4.07 × 10−8 mol L−1.
In order to verify the fine F− recognition properties coming from the supramolecular sensor P5N1Fe, a series of controlled experiments were carried out. As shown in Fig S13,† no separate component of the supramolecular sensor P5N1Fe, such as P5, N1, or P5N1, could sense F−. These results confirmed that the self-assembled supramolecular sensor P5N1Fe was the recognition host.
The reversible experiments also supported these results. By alternating the addition of Fe3+ and F− to the supramolecular sensor P5N1Fe water solution, the fluorescence emission of the tested solution performed alternate reviving and quenching processes (Fig. 8). This off–on–off switching process could be repeated several times with little fluorescent efficiency loss. These results confirmed that the F− recognition process is a competitive coordination process (Scheme 2).
 | ||
| Fig. 8 Fluorescence intensity reversible switching cycles of the P5N1 water solution (1 × 10−5 mol L−1) on alternate addition of Fe3+ and F− (λex = 376 nm). | ||
For ease of use, test kits for ions, such as ion responsive films (Fig. 9) or ion test strips (Fig. 10), based on these supramolecular sensors could be easily prepared. For example, by pouring the solution of these supramolecular sensors onto a clean glass surface and drying in air, we could obtain the corresponding ion responsive supramolecular films. Moreover, ion test strips could be fabricated by immersing filter papers into a water solution of the supramolecular sensors and then drying them in air.
As show in Fig. 9 and 10, these ion responsive films or test strips could act as convenient test kits for the detection of Fe3+ and F− in water. Moreover, as shown in Fig. 10, the P5N1 film has green fluorescence emission, such that when writing on the film with a writing brush dipped in Fe3+ water solution, a dark writing image appeared under UV conditions. Furthermore, the P5N1Fe film has no fluorescence, such that when writing on the film with a writing brush dipped in F− water solution, a brilliant green fluorescent writing image appeared. These fluorescent images could be erased by brushing F− or Fe3+ on the corresponding films again. Therefore, these films could act as security display materials.
In summary, by rationally introducing competitive binding interactions into a self-assembled cationic pillar[5]arene-based supramolecular sensor, water soluble supramolecular sensors P5N1 and P5N1Fe were successfully developed. These supramolecular sensors could sense Fe3+ and F− with high selectivity and sensitivity in water solution, respectively. It is worth mentioning that, due to the high hydration enthalpy of F−, the detection of F− in water is still a very difficult task, while P5N1Fe showed high selectivity for F− in water. Moreover, convenient test kits for ions and erasable security display materials based on these supramolecular sensors were prepared. Therefore, this is an efficient and simple way to develop chemosensors that could selectively detect ions in water.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (No. 21574104; 21161018; 21262032) and the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (IRT 15R56).Notes and references
- C. Caltagirone and P. A. Gale, Chem. Soc. Rev., 2009, 38, 520–563 RSC.
- K. P. Carter, A. M. Young and A. E. Palmer, Chem. Rev., 2014, 114, 4564–4601 CrossRef CAS PubMed.
- S. V. Krivovichev, O. Mentré, O. I. Siidra, M. Colmont and S. K. Filatov, Chem. Rev., 2013, 113, 6459–6535 CrossRef CAS PubMed.
- N. Busschaert, C. Caltagirone, W. V. Rossom and P. A. Gale, Chem. Rev., 2015, 115, 8038–8155 CrossRef CAS PubMed.
- J. Yoon, S. K. Kim, N. J. Singh and K. S. Kim, Chem. Soc. Rev., 2006, 35, 355–360 RSC.
- R. N. Dsouza, U. Pischel and W. M. Nau, Chem. Rev., 2011, 111, 7941–7980 CrossRef CAS PubMed.
- B. Hua, L. Shao, G. Yu and F. Huang, Chem. Commun., 2016, 52, 10016–10019 RSC.
- P. Wang, X. Yan and F. Huang, Chem. Commun., 2014, 50, 5017–5019 RSC.
- C. R. Wade, A. E. J. Broomsgrove, S. Aldridge and F. P. Gabbaï, Chem. Rev., 2010, 110, 3958–3984 CrossRef CAS PubMed.
- S. Jagtap, M. K. Yenkie, N. Labhsetwar and S. Rayalu, Chem. Rev., 2012, 112, 2454–2466 CrossRef CAS PubMed.
- Y. Zhou, J. F. Zhang and J. Yoon, Chem. Rev., 2014, 114, 5511–5571 CrossRef CAS PubMed.
- G. Yu, Z. Zhang, C. Han, M. Xue, Q. Zhou and F. Huang, Chem. Commun., 2012, 48, 2958–2960 RSC.
- T. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed.
- D. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang and H. Meier, Angew. Chem., Int. Ed., 2009, 48, 9721–9723 CrossRef CAS PubMed.
- P. J. Cragg and K. Sharma, Chem. Soc. Rev., 2012, 41, 597–607 RSC.
- M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294–1308 CrossRef CAS PubMed.
- G. Yu, D. Wu, Y. Li, Z. Zhang, L. Shao, J. Zhou, Q. Hu, G. Tang and F. Huang, Chem. Sci., 2016, 7, 3017–3024 RSC.
- K. Yang, Y. Pei, J. Wen and Z. Pei, Chem. Commun., 2016, 52, 9316–9326 RSC.
- K. Jie, Y. Zhou, Y. Yao, B. Shi and F. Huang, J. Am. Chem. Soc., 2015, 137, 10472–10475 CrossRef CAS PubMed.
- T. Ogoshi, S. Takashima and T. Yamagishi, J. Am. Chem. Soc., 2015, 137, 10962–10964 CrossRef CAS PubMed.
- B. Shi, K. Jie, Y. Zhou, J. Zhou, D. Xia and F. Huang, J. Am. Chem. Soc., 2016, 138, 80–83 CrossRef CAS PubMed.
- B. Shi, D. Xia and Y. Yao, Chem. Commun., 2014, 50, 13932–13935 RSC.
- Y. Chang, C. Hou, J. l. Ren, X. Xin, Y. Pei, Y. Lu, S. Cao and Z. Pei, Chem. Commun., 2016, 52, 9578–9581 RSC.
- X. Wu, Y. Li, C. Lin, X. Y. Hu and L. Wang, Chem. Commun., 2015, 51, 6832–6835 RSC.
- Y. Chang, K. Yang, P. Wei, S. Huang, Y. Pei, W. Zhao and Z. Pei, Angew. Chem., Int. Ed., 2014, 53, 13126–13130 CrossRef CAS PubMed.
- Y. Cao, X. Y. Hu, Y. Li, X. Zou, S. Xiong, C. Lin, Y. Z. Shen and L. Wang, J. Am. Chem. Soc., 2014, 136, 10762–10769 CrossRef CAS PubMed.
- Q. Duan, Y. Cao, Y. Li, X. Hu, T. Xiao, C. Lin, Y. Pan and L. Wang, J. Am. Chem. Soc., 2013, 135, 10542–10549 CrossRef CAS PubMed.
- T. Ogoshi, K. Kida and T. Yamagishi, J. Am. Chem. Soc., 2012, 134, 20146–20150 CrossRef CAS PubMed.
- M. Xue, Y. Yang, X. Chi, X. Yan and F. Huang, Chem. Rev., 2015, 115, 7398–7501 CrossRef CAS PubMed.
- W. Zheng, L. J. Chen, G. Yang, B. Sun, X. Wang, B. Jiang, G. Q. Yin, L. Zhang, X. Li, M. Liu, G. Chen and H. B. Yang, J. Am. Chem. Soc., 2016, 138, 4927–4937 CrossRef CAS PubMed.
- B. Jiang, J. Zhang, J. Q. Ma, W. Zheng, L. J. Chen, B. Sun, C. Li, B. W. Hu, H. Tan, X. Li and H. B. Yang, J. Am. Chem. Soc., 2016, 138, 738–741 CrossRef CAS PubMed.
- L.-L. Tan, H. Li, Y. C. Qiu, D.-X. Chen, X. Wang, R. Y. Pan, Y. Wang, S. X. A. Zhang, B. Wang and Y. W. Yang, Chem. Sci., 2015, 6, 1640–1644 RSC.
- Y. Fang, X. Yuan, L. Wu, Z. Peng, W. Feng, N. Liu, D. Xu, S. Li, A. Sengupta, P. K. Mohapatra and L. Yuan, Chem. Commun., 2015, 51, 4263–4266 RSC.
- J. Bi, X. Zeng, D. Tian and H. Li, Org. Lett., 2016, 18, 1092–1095 CrossRef CAS PubMed.
- Y. Ma, X. Ji, F. Xiang, X. Chi, C. Han, J. He, Z. Abliz, W. Chen and F. Huang, Chem. Commun., 2011, 47, 12340–12342 RSC.
- T. Ogoshi, M. Hashizume, T. Yamagishi and Y. Nakamoto, Chem. Commun., 2010, 46, 3708–3710 RSC.
- X. Y. Hu, X. Liu, W. Zhang, S. Qin, C. Yao, Y. Li, D. Cao, . Peng and L. Wang, Chem. Mater., 2016, 28, 3778–3788 CrossRef.
- Q. Lin, T. T. Lu, X. Zhu, T. B. Wei, H. Li and Y. M. Zhang, Chem. Sci., 2016, 7, 5341–5346 RSC.
- Q. Lin, T.-T. Lu, J.-C. Lou, G.-Y. Wu, T.-B. Wei and Y.-M. Zhang, Chem. Commun., 2015, 51, 12224–12227 RSC.
- Q. Lin, T. T. Lu, X. Zhu, B. Sun, Q. P. Yang, T.-B. Wei and Y.-M. Zhang, Chem. Commun., 2015, 51, 1635–1638 RSC.
- Q. Lin, B. Sun, Q. P. Yang, Y. P. Fu, X. Zhu, T. B. Wei and Y. M. Zhang, Chem.–Eur. J., 2014, 20, 11457–11462 CrossRef CAS PubMed.
- Q. Lin, X. Liu, T. B. Wei and Y. M. Zhang, Chem.–Asian J., 2013, 8, 3015–3021 CrossRef CAS PubMed.
- T. B. Wei, J. F. Chen, X. B. Cheng, H. Li, Q. Lin, H. Yao and Y. M. Zhang, RSC Adv., 2016, 6, 65898–65901 RSC.
- G. Y. Wu, B. B. Shi, Q. Lin, H. Li, Y. M. Zhang, H. Yao and T. B. Wei, RSC Adv., 2015, 5, 4958–4963 RSC.
- S. Tsuzuki, K. Honda, T. Uchimaru, M. Mikami and K. Tanabe, J. Am. Chem. Soc., 2002, 124, 104–112 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra23878e |
| This journal is © The Royal Society of Chemistry 2016 |