Green preparation of d-tryptophan imprinted self-supported membrane for ultrahigh enantioseparation of racemic tryptophan
Abstract
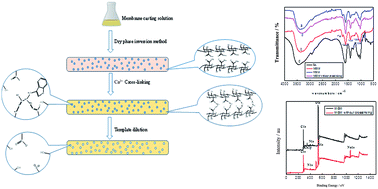
Enantioseparation of chiral compounds is important for pharmaceutical production. Chiral resolution of racemic tryptophan has attracted increasing attention. Traditional separation methods have the disadvantages of being high cost, energy intensive and environmentally unfriendly. Membrane permeation seems promising for application in the enantioseparation of racemic tryptophan with the advantages of energy efficiency and no additives. However, lots of organic compounds are commonly used for the preparation of various membranes for enantioseparation of racemic tryptophan. Therefore, we prepared a molecularly imprinted self-supported membrane (MISM) using a green and clean method with natural polymer sodium alginate (SA) as the functional polymer, water as the solvent and CaCl2 as the crosslinking agent. The crosslinking by CaCl2 was confirmed by attenuated total reflection Fourier transform infrared (ATR-FTIR) and X-ray photoelectron spectroscopy (XPS) analyses. The surface and internal structures of the MISM and non-imprinted self-supported membrane (NISM) were observed by atomic force microscope (AFM) and scanning electron microscope (SEM). The addition of a template molecule can significantly improve the MISM surface roughness. The compact structures of MISM and NISM were confirmed by the cross-sectional images. The thermo stability of MISM was studied. In addition, the effects of pH and crosslinking time on the swelling behavior of MISM were investigated. A high concentration of OH− in alkali solution can remarkably weaken the electrostatic interaction between Ca2+ and COO−. The effects of preparation conditions, permeation conditions and concentration polarization on the pressure-driven permeation performance of MISM were investigated. Ultrahigh enantioseparation performance in 99% ee of the permeation solution can be obtained under mild conditions. Reducing the concentration polarization by increasing the environmental temperature and continuous stirring is beneficial to obtaining ultrahigh ee. Comparison with previous studies on pressure-driven permeation performance indicates that the proposed green and clean preparation method for a molecularly imprinted self-supported membrane is not only environmentally friendly but also efficient in obtaining ultrahigh ee.

 Please wait while we load your content...
Please wait while we load your content...