Dual signal amplification by an “on-command” pure DNA hydrogel encapsulating HRP for colorimetric detection of ochratoxin A†
Lu Zhou,
Na Sun,
Lijun Xu,
Xing Chen,
Hui Cheng,
Jine Wang* and
Renjun Pei*
Key Laboratory of Nano-Bio Interface, Division of Nanobiomedicine, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou, 215123, China. E-mail: rjpei2011@sinano.ac.cn; jewang2012@sinano.ac.cn
First published on 30th November 2016
Abstract
A pure DNA hydrogel, consisting of two kinds of Y-scaffold nucleic acid subunits and the aptamer domain of ochratoxin A, undergoes a switchable gel-to-sol transition in the presence of ochratoxin A. The target molecule stimulated the disassembly of the DNA hydrogel to release the encapsulated HRP, which triggered the reaction of H2O2 and ABTS for dual signal amplification of colorimetric detection.
In recent decades, DNA has emerged as a new kind of biomaterial for biomedical applications which has taken on versatile roles in nanometer fields.1 DNA possesses many distinguished properties, including its biocompatibility, target recognition ability and 3-D structure controllability.2–6 Taking advantage of these unique properties, a kind of novel DNA material, a DNA-based hydrogel, has been created with properties derived from the 3-D structure controllability and molecular recognition ability.7–9 Numerous research efforts have shown the development and applications of hydrogel materials.10–13 Specifically, the development of a stimuli-responsive switchable hydrogel has attracted much research interest.14,15 Different stimulus to trigger switchable hydrogel transitions were used, including pH,16 light,17 temperature,18,19 and host–guest supramolecular interactions.20,21 Different applications of hydrogels and stimuli-responsive hydrogels were suggested, including controlled drug delivery and release,22,23 tissue engineering,10,24 stimuli-controlled pumps or valves,25,26 and sensors or actuators.27,28 One specific class of hydrogel is DNA-based hydrogel.6,29 Two general strategies were implemented to develop DNA-based hydrogels. One approach involves tethering of nucleic acids to hydrophilic polymer and hydrogel formation through hybridization of the DNA chains.30 Tan group has demonstrated a general design for a colorimetric visual detection platform based on an aptamer cross-linked hydrogel.31 They also reviewed the progress of aptamers incorporating into hydrogel systems to enable colorimetric detection, controlled drug release, and targeted cancer therapy.32 The catalytic one-cycle dissociation of hydrogels by enzymes or DNAzymes33 was demonstrated, and cyclic gel-to-solution transitions using switchable i-motif34 or G-quadruplex35 crosslinking motif were also reported. Different applications of pure DNA hydrogels were suggested including the removal of hazardous ions (e.g. Hg2+),36 sensing,37 inscription of structural information,38 switchable fluorescence properties,39 and catalytic DNAzyme functions.35 Yang group described a design by combining glucoamylase-trapped aptamer crosslinked hydrogel with a personal glucose meter (PGM) for portable and quantitative detection of non-glucose targets.9
By the other method, pure nucleic acid units were crosslinked by hybridization to form the hydrogel. The forming of pure DNA hydrogel takes the advantage of the self-assembly of DNA into the ordered structure. Pure DNA hydrogel has many distinguished properties, including its biocompatibility and easy synthesis. Luo and his co-workers have developed a method to construct pure DNA hydrogel by DNA self-assembly and further enzymatic ligation.40 Liu group built a versatile strategy to prepare DNA hydrogels with designable build block Y-scaffold and linker.41 Aptamers, as a novel class of molecular probes for molecular recognization, have attracted much attention in recent years. Aptamers are short structured, single-stranded DNA or RNA that can recognize their targets with high affinity and specificity42 The integration of DNA aptamer sequence into DNA hydrogel has great potential to enable pure DNA hydrogel with excellent recognition ability which is responsive to the target molecule due to the high sensitive and selective interaction between the aptamer and the target molecule. Ju group reported an aptamer-functionalized DNA hydrogel, which was designed as a specifically target responsive switchable material for thrombin detection by entrapping Au nanoparticles for color change.7 Nonetheless, to date there has been no report on small molecule detection by using small molecule–aptamer interaction to stimulate the formation and dissociation of pure DNA hydrogel. Here we designed three build blocks integrating the aptamer sequence of a small molecule to form pure DNA hydrogel for sensitive detection of the small molecule.
Ochratoxin A (OTA) is a toxin produced by the Aspergillus ochraceus and Penicillium verrucosum, which is widely present in foods, including corn barley, oats, wheat, rye, rice, vegetables and other crops.43–45 Extensive studies have been reported that the major violation sites of OTA are liver and kidney of mammalian species and it could further cause carcinogenic, hepatotoxic, nephrotoxic, and immunotoxic effects on most mammalian species.46,47 Considering the potential toxic effects of OTA, the development of reliable and rapid methods for measurement of OTA in food samples is of great practical importance for food safety. Here, OTA as a model small molecule, the biosensor based on aptamer-target recognition to modulate DNA hydrogel system was developed.
In the present study, we introduce two kinds of Y-scaffold DNA subunits and the aptamer domain of target molecule, to prepare target-dependent switchable DNA hydrogel that encapsulates HRP for secondary signal amplification by enzyme reaction immediately when mixing with H2O2, 2,2′-azino bis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS2−) after the release of the encapsulated HRP in the bulk solution.
The aptamer-crosslinked hydrogel is schematically presented in Fig. 1. The DNA hydrogel is assembled by three building blocks: Y-scaffold DNA subunit Y1-Scaffold (Y1-S), Y2-Scaffold (Y2-S) and the aptamer of target molecule as linker DNA (L-apt). Y1-S is formed from three single-strand DNAs (ssDNAs), with each 37-mer ssDNA containing two functional domains: “stick end”, which is a 9-mer complementing part from 3′ end of L-apt (marked in pink in Table S1†) and a 28-mer domain for formation of the double-strand Y-scaffold (marked in black, Table S1†). The 28-mer domain has been designed to have two half-complimentary sequences, so that equal amount of the three DNA strands will hybridize to each other to produce the Y-scaffold carrying three “stick ends”. The same way, Y2-S is formed having “stick end”, which is a 12-mer complementing part from 5′-end of L-apt (marked in green in Table S1†) and a 28-mer for formation of double-strand part of Y-scaffold. Since the “stick ends” in Y1-S and Y2-S were complementary to L-apt from 3′ end and 5′ end respectively, the DNA hydrogel was formed by the cross-linking hybridization between Y-scaffolds and linker DNA (L-apt). The rigid space formed in DNA hydrogel could encapsulate the signal molecules such as HRP, which can trigger enzyme reaction when it is released and transferred into a solution containing hydrogen peroxide (H2O2) and 2,2′-azino bis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS2−). When adding the buffer to the hydrogel, two colorless layers would be observed. Upon adding target molecule, ochratoxin A (OTA) which is the recognition molecule of the L-apt, the collapse and dissolution of the DNA hydrogel would happen. Thus HRP was released from the DNA hydrogel to the upper solution, for visual detection of the color change.
 | ||
| Fig. 1 Schematic diagram of the dual signal amplification system for OTA detection by on-command pure DNA hydrogel encapsulating HRP. | ||
Our first experiment was to determine whether we could achieve the designed Y1-S and Y2-S. Agarose gel electrophoresis was used to verify the formation of the Y-scaffolds (Fig. 2B). Y11, Y12, Y13, Y21, Y22 and Y23 were also loaded as controls. Here, the molar ratio of ssDNA was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in assembling each Y-scaffold. Compared with the single-strand DNAs at shorter electrophoresis distance, the Y1-S and Y2-S were indeed formed as designed and clean single bands indicate that the assembling process was efficient. After the Y1-S, Y2-S and L-apt were mixed at the molar ration of 1
1 in assembling each Y-scaffold. Compared with the single-strand DNAs at shorter electrophoresis distance, the Y1-S and Y2-S were indeed formed as designed and clean single bands indicate that the assembling process was efficient. After the Y1-S, Y2-S and L-apt were mixed at the molar ration of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the DNA hydrogel was successfully synthesized by assembly of Y-scaffolds with linker DNA (Fig. 1). The hydrogel forming was very fast in 10 to 20 seconds. Compared with the solution of Y1-S (Fig. 2A), the formed DNA gel still remained at the bottom of the EP tube, indicating the loss of the fluidity. To better visualize the gelling formation and optimize the experiment condition, a series concentrations of HRP solution were employed as signal agent in the presence of H2O2 and ABTS2− (Fig. S2†).
1, the DNA hydrogel was successfully synthesized by assembly of Y-scaffolds with linker DNA (Fig. 1). The hydrogel forming was very fast in 10 to 20 seconds. Compared with the solution of Y1-S (Fig. 2A), the formed DNA gel still remained at the bottom of the EP tube, indicating the loss of the fluidity. To better visualize the gelling formation and optimize the experiment condition, a series concentrations of HRP solution were employed as signal agent in the presence of H2O2 and ABTS2− (Fig. S2†).
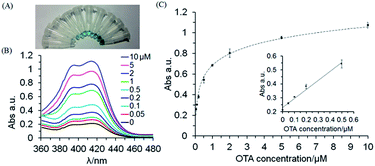
Due to the strong affinity between aptamer and OTA target, the DNA hydrogel would collapse and dissolve in the presence of OTA molecules. In this way, HRP was released into the upper solution, facilitating a visual signal-amplified detection method of OTA. The optimized DNA hydrogel with entrapped HRP was investigated for the visual response to different concentrations of OTA (Fig. 3A). The color tended to be deeper as the concentration of target increased, which is consistent with the increase in absorbance at approximate 418 nm from UV-vis spectra (Fig. 3B). The calibration plot showed a good linear relationship between the absorbance intensity and OTA concentration in a range of 0.05 to 0.5 μM with a correlation coefficient of 0.995 (inset in Fig. 3C). The detection limit at 3σ was 10.8 nM and the quantitation limit at 10σ was 36 nM. The low concentration for visual detection using the present hydrogel system may be attributed to the stimuli-sensitive structure of self-assembled DNA and the recognition of aptamer for OTA in a single-stand structure. However, the reaction conditions such as the concentrations of H2O2 and ABTS2− could be systematically optimized for more sensitive detection of OTA.
This method with high sensitivity is promising in practical applications. Wine was purchased from the local supermarket as the real food sample. As shown in Table 1, it was indicated that the system of “on-command” pure DNA hydrogel can be applied to detect concentration of OTA in complex food commodity. The recovery of OTA from 0.1–0.5 μM added in wine sample was from 97.6%–102.2%. It demonstrated the potential application of this OTA detection system for real food samples.
Conclusions
The pure DNA hydrogel as a dual signal amplification system was successfully applied in bioanalysis by loading HRP as signal agents. Two kinds of Y-scaffold DNA subunits and the aptamer domain of ochratoxin A, were utilized to prepare target-dependent switchable DNA hydrogel. The recognization of OTA with its aptamer stimulated the disassembly of DNA hydrogel to release the encapsulated HRP, which triggered the reaction of H2O2 and ABTS2− for dual signal amplification of sensitive colorimetric detection.Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 31301495, 21628502, 21275156, 21507156), the Natural Science Foundation of Jiangsu Province (BK20130351) and the Science and Technology Foundation of Suzhou (No. SYG201526).Notes and references
- D. Yang, M. R. Hartman, T. L. Derrien, S. Hamada, D. An, K. G. Yancey, R. Cheng, M. Ma and D. Luo, DNA materials: bridging nanotechnology and biotechnology, Acc. Chem. Res., 2014, 47, 1902–1911 CrossRef CAS PubMed.
- N. C. Seeman, DNA nanotechnology: novel DNA constructions, Annu. Rev. Biophys. Biomol. Struct., 1998, 27, 225–248 CrossRef CAS PubMed.
- A. Condon, Designed DNA molecules: principles and applications of molecular nanotechnology, Nat. Rev. Genet., 2006, 7, 565–575 CrossRef CAS PubMed.
- U. Feldkamp and C. M. Niemeyer, Rational design of DNA nanoarchitectures, Angew. Chem., Int. Ed., 2006, 45, 1856–1876 CrossRef CAS PubMed.
- A. V. Pinheiro, D. Han, W. M. Shih and H. Yan, Challenges and opportunities for structural DNA nanotechnology, Nat. Nanotechnol., 2011, 6, 763–772 CrossRef CAS PubMed.
- Y. H. Roh, R. C. Ruiz, S. Peng, J. B. Lee and D. Luo, Engineering DNA-based functional materials, Chem. Soc. Rev., 2011, 40, 5730–5744 RSC.
- L. Zhang, J. Lei, L. Liu, C. Li and H. Ju, Self-assembled DNA hydrogel as switchable material for aptamer-based fluorescent detection of protein, Anal. Chem., 2013, 85, 11077–11082 CrossRef CAS PubMed.
- W. Guo, X. J. Qi, R. Orbach, C. H. Lu, L. Freage, I. Mironi-Harpaz, D. Seliktar, H. H. Yang and I. Willner, Reversible Ag(+)-crosslinked DNA hydrogels, Chem. Commun., 2014, 50, 4065–4068 RSC.
- L. Yan, Z. Zhu, Y. Zou, Y. Huang, D. Liu, S. Jia, D. Xu, M. Wu, Y. Zhou, S. Zhou and C. J. Yang, Target-responsive “sweet” hydrogel with glucometer readout for portable and quantitative detection of non-glucose targets, J. Am. Chem. Soc., 2013, 135, 3748–3751 CrossRef CAS PubMed.
- K. Y. Lee and D. J. Mooney, Hydrogels for tissue engineering, Chem. Rev., 2001, 101, 1869–1880 CrossRef CAS PubMed.
- N. A. Peppas, J. Z. Hilt, A. Khademhosseini and R. Langer, Hydrogels in biology and medicine: from molecular principles to bionanotechnology, Adv. Mater., 2006, 18(11), 1345–1360 CrossRef CAS.
- D. Seliktar, Designing cell-compatible hydrogels for biomedical applications, Science, 2012, 336, 1124–1128 CrossRef CAS PubMed.
- J. Yuan, D. Wen, N. Gaponik and A. Eychmüller, Enzyme-Encapsulating Quantum Dot Hydrogels and Xerogels as Biosensors: Multifunctional Platforms for Both Biocatalysis and Fluorescent Probing, Angew. Chem., Int. Ed., 2013, 52, 976–979 CrossRef CAS PubMed.
- A. Döring, W. Birnbaum and D. Kuckling, Responsive hydrogels-structurally and dimensionally optimized smart frameworks for applications in catalysis, micro-system technology and material science, Chem. Soc. Rev., 2013, 42, 7391–7420 RSC.
- A. B. Imran, T. Seki and Y. Takeoka, Recent advances in hydrogels in terms of fast stimuli responsiveness and superior mechanical performance, Polym. J., 2010, 42, 839–851 CrossRef.
- S. R. Haines and R. G. Harrison, Novel resorcinarene-based pH-triggered gelator, Chem. Commun., 2002, 2846–2847 RSC.
- J. J. de Jong, L. N. Lucas, R. M. Kellogg, J. H. van Esch and B. L. Feringa, Reversible optical transcription of supramolecular chirality into molecular chirality, Science, 2004, 304, 278–281 CrossRef CAS PubMed.
- K. Kuroiwa, T. Shibata, A. Takada, N. Nemoto and N. Kimizuka, Heat-set gel-like networks of lipophilic Co(II) triazole complexes in organic media and their thermochromic structural transitions, J. Am. Chem. Soc., 2004, 126, 2016–2021 CrossRef CAS PubMed.
- B. Jeong, S. W. Kim and Y. H. Bae, Thermosensitive sol–gel reversible hydrogels, Adv. Drug Delivery Rev., 2002, 54, 37–51 CrossRef CAS PubMed.
- K. Murata, M. Aoki, T. Nishi, A. Ikeda and S. Shinkai, New cholesterol-based gelators with light-and metal-responsive functions, J. Chem. Soc., Chem. Commun., 1991, 1715–1718 RSC.
- J. B. Beck and S. J. Rowan, Multistimuli, multiresponsive metallo-supramolecular polymers, J. Am. Chem. Soc., 2003, 125, 13922–13923 CrossRef CAS PubMed.
- M. E. Byrne, K. Park and N. A. Peppas, Molecular imprinting within hydrogels, Adv. Drug Delivery Rev., 2002, 54, 149–161 CrossRef CAS PubMed.
- J. Z. Hilt and M. E. Byrne, Configurational biomimesis in drug delivery: molecular imprinting of biologically significant molecules, Adv. Drug Delivery Rev., 2004, 56, 1599–1620 CrossRef CAS PubMed.
- J. A. Rowley, G. Madlambayan and D. J. Mooney, Alginate hydrogels as synthetic extracellular matrix materials, Biomaterials, 1999, 20, 45–53 CrossRef CAS PubMed.
- D. J. Beebe, J. S. Moore, J. M. Bauer, Q. Yu, R. H. Liu, C. Devadoss and B.-H. Jo, Functional hydrogel structures for autonomous flow control inside microfluidic channels, Nature, 2000, 404, 588–590 CrossRef CAS PubMed.
- T. A. Kapur and M. S. Shoichet, Immobilized concentration gradients of nerve growth factor guide neurite outgrowth, J. Biomed. Mater. Res., Part A, 2004, 68, 235–243 CrossRef PubMed.
- C. Ruan, K. Zeng, O. K. Varghese and C. A. Grimes, Magnetoelastic Immunosensors: Amplified Mass Immunosorbent Assay for Detection of Escherichia c oli O157: H7, Anal. Chem., 2003, 75, 6494–6498 CrossRef CAS PubMed.
- J. H. Holtz and S. A. Asher, Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials, Nature, 1997, 389, 829–832 CrossRef CAS PubMed.
- J. Liu, Oligonucleotide-functionalized hydrogels as stimuli responsive materials and biosensors, Soft Matter, 2011, 7, 6757–6767 RSC.
- T. Liedl, H. Dietz, B. Yurke and F. Simmel, Controlled Trapping and Release of Quantum Dots in a DNA-Switchable Hydrogel, Small, 2007, 3, 1688–1693 CrossRef CAS PubMed.
- H. Yang, H. Liu, H. Kang and W. Tan, J. Am. Chem. Soc., 2008, 130, 6320–6321 CrossRef CAS PubMed.
- Z. Zhu, C. Wu, H. Liu, Y. Zou, X. Zhang, H. Kang, C. J. Yang and W. Tan, An aptamer cross-linked hydrogel as a colorimetric platform for visual detection, Angew. Chem., Int. Ed. Engl., 2010, 49, 1052–1056 CrossRef CAS.
- W. YunáZhang and C. JamesáYang, DNAzyme crosslinked hydrogel: a new platform for visual detection of metal ions, Chem. Commun., 2011, 47, 9312–9314 RSC.
- E. Cheng, Y. Xing, P. Chen, Y. Yang, Y. Sun, D. Zhou, L. Xu, Q. Fan and D. Liu, A pH-triggered, fast-responding DNA hydrogel, Angew. Chem., 2009, 121, 7796–7799 CrossRef.
- C.-H. Lu, X.-J. Qi, R. Orbach, H.-H. Yang, I. Mironi-Harpaz, D. Seliktar and I. Willner, Switchable catalytic acrylamide hydrogels cross-linked by Hemin/G-Quadruplexes, Nano Lett., 2013, 13, 1298–1302 CrossRef CAS PubMed.
- N. Dave, M. Y. Chan, P.-J. J. Huang, B. D. Smith and J. Liu, Regenerable DNA-functionalized hydrogels for ultrasensitive, instrument-free mercury(II) detection and removal in water, J. Am. Chem. Soc., 2010, 132, 12668–12673 CrossRef CAS PubMed.
- K. A. Joseph, N. Dave and J. Liu, Electrostatically directed visual fluorescence response of DNA-functionalized monolithic hydrogels for highly sensitive Hg2+ detection, ACS Appl. Mater. Interfaces, 2011, 3, 733–739 CAS.
- J. B. Lee, S. Peng, D. Yang, Y. H. Roh, H. Funabashi, N. Park, E. J. Rice, L. Chen, R. Long and M. Wu, A mechanical metamaterial made from a DNA hydrogel, Nat. Nanotechnol., 2012, 7, 816–820 CrossRef CAS PubMed.
- W. Guo, R. Orbach, I. Mironi-Harpaz, D. Seliktar and I. Willner, Fluorescent DNA Hydrogels Composed of Nucleic Acid-Stabilized Silver Nanoclusters, Small, 2013, 9, 3748–3752 CrossRef CAS PubMed.
- S. H. Um, J. B. Lee, N. Park, S. Y. Kwon, C. C. Umbach and D. Luo, Enzyme-catalysed assembly of DNA hydrogel, Nat. Mater., 2006, 5(10), 797–801 CrossRef CAS PubMed.
- Y. Xing, E. Cheng, Y. Yang, P. Chen, T. Zhang, Y. Sun, Z. Yang and D. Liu, Self-assembled DNA hydrogels with designable thermal and enzymatic responsiveness, Adv. Mater., 2011, 23, 1117–1121 CrossRef CAS PubMed.
- H. Wang, J. Wang and N. Sun, et al. Selection and Characterization of Malachite Green Aptamers for the Development of Light-up Probes, Chem. Select, 2016, 1(8), 1571–1574 CAS.
- J. A. Cruz-Aguado and G. Penner, Fluorescence polarization based displacement assay for the determination of small molecules with aptamers, Anal. Chem., 2008, 80, 8853–8855 CrossRef CAS PubMed.
- N. Ahmed, M. Farag, K. Soliman, A. Abdel-Samed and K. M. Naguib, Evaluation of methods used to determine ochratoxin a in coffee beans, J. Agric. Food Chem., 2007, 55, 9576–9580 CrossRef CAS PubMed.
- R. Imperato, L. Campone, A. L. Piccinelli, A. Veneziano and L. Rastrelli, Survey of aflatoxins and ochratoxin a contamination in food products imported in Italy, Food Control, 2011, 22, 1905–1910 CrossRef CAS.
- S. Duarte, A. Pena and C. Lino, A review on ochratoxin A occurrence and effects of processing of cereal and cereal derived food products, Food Microbiol., 2010, 27, 187–198 CrossRef CAS PubMed.
- A. Lühe, H. Hildebrand, U. Bach, T. Dingermann and H.-J. Ahr, A new approach to studying ochratoxin A (OTA)-induced nephrotoxicity: expression profiling in vivo and in vitro employing cDNA microarrays, Toxicol. Sci., 2003, 73, 315–328 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experiment procedures and supplementary data. See DOI: 10.1039/c6ra23462c |
| This journal is © The Royal Society of Chemistry 2016 |