Structure and property optimization of perfluorinated short side chain membranes for hydrogen fuel cells using orientational stretching†
Abstract
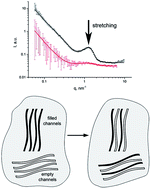
Small-angle neutron scattering has been applied to study the structure peculiarities of perfluorinated proton conducting polymer samples containing sulfonic groups of the Aquivion® type in dry and moistened conditions, which differ from Nafion® type membranes by the length of the side chains with sulfonic acid groups. The fine structure of the membranes is revealed, which is based on a system of regular proton conducting channels in the perfluorinated polymer matrix. The way this fine structure changes was determined as a function of the equivalent weight of the membrane, and the relation of these changes with proton conductivity value is established. The neutron contrast variation method enabled us to study the effect of orientational stretching on the fine structure. It was found that stretching is accompanied by an increase in proton conductivity due to changes in the fine structure of the channel system. Our investigations confirm that a reduction in the side chain length affects the fine structure of the perfluorinated proton conducting membranes, which is accompanied by an improvement in their performance in hydrogen fuel cells. Therefore, Aquivion® type systems will allow to reduce and possibly remove the existing operational restrictions of Nafion®.


 Please wait while we load your content...
Please wait while we load your content...