Fluorescent bio-nanocomposites based on chitosan reinforced hemicyanine dye-modified montmorillonite†
M. E. M. Mekhzoumab,
E. M. Essassiab,
A. Qaissa and
R. Bouhfid*a
aMoroccan Foundation for Advanced Science, Innovation and Research (MAScIR), Institute of Nanomaterial and Nanotechnology (NANOTECH), Rabat Design, Rue Mohamed El Jazouli, Madinat Al Irfane, 10100 Rabat, Morocco. E-mail: r.bouhfid@mascir.com
bLaboratoire de Chimie Organique Hétérocyclique, Université Mohammed V, Faculté des Sciences, Av. Ibn Battouta, BP 1014, Rabat, Morocco
First published on 16th November 2016
Abstract
The present investigation describes the synthesis and detailed characterization of novel fluorescent bio-nanocomposite films of chitosan reinforced by hemicyanine dye-modified montmorillonite (MMT–HD) using a solvent-casting method. A series of hemicyanine dyes (HD) have been synthesized by catalyst-free and solvent-free conditions in high yields. These organic compounds were intercalated within the interlayer gallery of montmorillonite providing an increase in the basal spacing and affording inorganic–organic host–guest materials. The fundamental properties of these dyes as well as their intercalated MMT were studied by X-ray, FTIR, and thermal analysis, completed by photophysical properties studies. The results showed that the guest species (HD) are successfully intercalated and stacked as J-aggregates (head-to-tail aggregates) into the host layer of MMT, which is confirmed by spectral characterization. Improvement in thermal stability of the intercalated MMT was indicated by results from thermal analysis compared to the pure dye. Next, the resulting organo-modified clays were dispersed within the chitosan biopolymer. The obtained bio-films were investigated and characterized with varying content of clay (1, 2, 5 and 10 wt%). The structural, hygroscopic (hydrophobicity and water solubility) and fluorescence properties of the resulting materials were evaluated. Results demonstrate that the incorporation of MMT–HD into chitosan improves the hygroscopic properties of the chitosan film, thus showing potential for active food packaging materials. In addition, the solid state fluorescence spectra of the bio-films have shown a significant fluorescence emission at room temperature with increasing clay content.
Introduction
In the last few decades, science and technology has started to move in the direction of renewable materials that are environmentally friendly and sustainable.1 This evolution motivates academic and industrial research to develop novel materials from alternative resources, with lower energy consumption, biodegradable and non-toxic to the environment.2,3 These materials are often formulated with natural biopolymer sources, such as starch, cellulose, chitin or biodegradable synthetic polymers namely, polycaprolactone and polylactic acid, capable of forming a cohesive and continuous matrix.4,5 Nowadays, biopolymers became the subject of intensive studies for the development of biodegradable films.6,7 Unfortunately, the usage of biopolymers as food packaging materials has drawbacks such as poorer mechanical, thermal, and barrier properties as compared to the conventional non-biodegradable materials made from petroleum.Among all the natural polymers, chitosan has increased much attention recent years for packaging applications due to its intrinsic properties that depend on environmental variables.8 Chitosan, a natural linear polysaccharide obtained from crustaceans consisting mainly of β-(1,4)-linked 2-deoxy-2-amino-D-glucopyranose units and partially of β-(1,4)-linked 2-deoxy-2-acetamido-D-glucopyranose, is the deacetylated derivative of chitin, which is the second most abundant natural polymer after cellulose.9 Chitosan has some unique properties including biocompatibility, biodegradability, hydrophilicity, non-toxicity, non-antigenicity, bio-adherence and cell affinity. It is the only positively charged naturally occurring polysaccharide which strongly interacts with negatively charged entities.10 Owing to its low cost, large-scale availability and antimicrobial activity, chitosan has been widely used in food and pharmaceutical industry.11 Additionally, this polysaccharide has been extensively studied in the field of biomaterials; particularly on chitosan based functional nanomaterials.12 Nevertheless, poor mechanical and gas barrier properties, and weak water resistance limits its application particularly in the presence of water and humidity.13 In an attempt to obtain chitosan with an ultimate property, different nano-sized reinforcements were incorporated.14,15 The presences of nanosized filler result in a very promising materials since they exhibit enhanced properties with preservation of the material biodegradability without eco-toxicity.16 At present, the addition of layered silicates in particular montmorillonite (MMT) in chitosan has been extensively studied.17,18 Unfortunately, the incompatible nature of the two components discounts the desirable properties of the composite. For this reason, clay must be treated before used and organomodifying the surface properties of the clay with surfactants maybe a highly effective method. In this respect, the compatibility between the inorganic clay and organic chitosan is enhanced. Recent research has indicated that incorporation of organically modified MMT shows much promise for chitosan-based polymer nanocomposites in terms of improvements their performance in various properties.19 There are several reports about the nanocomposites based on chitosan. In light of these facts. Günister et al.20 found that both the structure of chitosan/MMT nanocomposites and its thermal stability are strongly affected by the solvent-casting procedure used. On the other hand. Darder et al.21 employed cationic biopolymer chitosan into MMT–Na+, providing a –NH3+ functional group for the anion exchange site as an anion sensor. The d spacing of pristine clay increased from 1.20 nm to 2.09 nm at the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chitosan–clay ratio. In another recent study, Sahoo et al.22 prepared a blend of chitosan, polycaprolactone (80
1 chitosan–clay ratio. In another recent study, Sahoo et al.22 prepared a blend of chitosan, polycaprolactone (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 ratio), and Cloisite 30B by solution mixing. The results indicated that the interaction of Cloisite 30B occurs together with exfoliation and the extent of interaction increases with an increase in clay concentration from 1 to 5 wt%. However, the above interesting finding was attributed and limited to the evolved structure.
20 ratio), and Cloisite 30B by solution mixing. The results indicated that the interaction of Cloisite 30B occurs together with exfoliation and the extent of interaction increases with an increase in clay concentration from 1 to 5 wt%. However, the above interesting finding was attributed and limited to the evolved structure.
Hitherto, the design of materials with fluorescent properties has raised huge attention due to their potential applications in numerous advanced technologies.23 While, a number of fluorescent nanomaterials, such as quantum dots (QDs),24 upconversion nanoparticles (UPNPs),25 polymer-based fluorescent nanoparticles and dye-doped silica nanoparticles (DDSNs), have already been reported in the literature.26,27 Further, the intercalation of organic guest species into smectites is a way of constructing ordered inorganic–organic assembly with unique microstructures controlled by host–guest and guest–guest interactions. Molecules with appropriate structural features upon intercalation can preserve/exhibit a number of specific properties such as photoluminescence,28 photochromism,29 optical nonlinearity (NLO)30 and among others.31 Along these lines, several reports have been published on the photophysical properties of MMT-based dye nanocomposites with cationic azobenzene,32 pyrene,33 β-carotene,34 rhodamine B35 and coumarin36 types of dye chromophores. Arbeloa et al.37 have studied the aggregation of several rhodamine dyes adsorbed into different smectite-type clays in dilute aqueous suspensions, depending on the nature of the clay and the hydrophobic character of the dye, by spectroscopic analysis. However, the incorporation of organic dyes into chitosan/clay nanocomposites, is neither contemplated nor disclosed. Historically, a lot of classes of fluorescent organic dyes have found their successful application in science and technology.38 As a popular example, hemicyanine dye and its derivatives having donor–π–acceptor structure are widely applied in different areas of technology due to their diversified properties.39 They are commonly applied to lasers, electronics, and nonlinear optics.40 Several such systems containing benzothiazole-derived dyes with a D–π–A setup have already been synthesized and studied intensively for several decades due to their potential applications in molecular electronics and biological fluorescence probing.41 Taking into account these antecedents, in this study, we herein reported a facile and efficient synthesis of series of 2-styrylbenzothiazolium hemicyanine dyes and employed as an intercalating agent for the preparation of organophilic clay through cation exchange reaction. Afterward, the prepared MMT–HD have been mixed with chitosan (CS) via solvent-casting method to produce CS/MMT–HD bio-films. The incorporation of MMT–HD into chitosan enhances both the fluorescence and hygroscopic properties of the bio-films. By combining the advantages of the good properties of chitosan, montmorillonite and hemicyanine dyes, consequently, the bio-film can be developed to fulfill the properties needed as fluorescence and packaging materials.
Results and discussion
Synthetic procedure and structure details of hemicyanine dye
Our target structures can be synthesized into two steps: (i) preparation of 3-ethyl-2-methylbenzothiazolium salt 2 and (ii) preparation of 3-ethyl-2-(p/o-substituted styryl)benzothiazol-3-ium iodide HDa–d, where the corresponding dyes are connected via an ethenylene spacer. The structures of the 4 styryl benzothiazolium dyes synthesized have one type of electron acceptor group (benzothiazole) but differ in a structure of phenyl moiety substituent (Scheme 1). As indicated in Scheme 2, the first step concerns the formation of the benzothiazolium salt by alkylation with the corresponding iodoethane, readily available using DMF as solvent to afford the compound close to 92% yield. The second one was carried out under catalyst-free and solvent-free conditions between 3-ethyl-2-methylbenzothiazolium iodide and series of p/o-substituted benzaldehydes. The hemicyanine dye structures were obtained in good to high yields independent of substituents on the phenyl rings. Furthermore, product isolation does not require purification by column chromatography.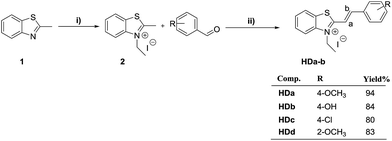 | ||
| Scheme 2 Synthetic pathway for preparation of 3-ethyl-2-(p/o-substituted styryl)benzothiazolium iodides HDa–d. Reagents and conditions: (i) C2H5I, DMF, reflux; (ii) catalytic free and solvent-free. | ||
A general route for the synthesis of the prepared dyes is shown in Scheme 2.
The prepared final compounds HDa–d have “push–pull” structure characterized by the electron-donor and electron-acceptor groups interacting through π-conjugated spacer, the benzothiazole based styryl dyes studied show several specific properties that are similar to other styryl dyes reported in literature.42 The structures of dyes were unambiguously confirmed by 1H and 13C NMR, mass spectrometry and were assigned on the basis of their spectral data in comparison to those reported in other literature. The most characteristic signals in the 1H NMR spectra of this family of compounds were those corresponding to the CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH ethene protons at 7.86–8.24 ppm with coupling constants ≈15 Hz indicating their E conformation, and the N–CH2 protons of the benzothiazolium ring which resonate between 4.90 and 4.98 ppm. These data confirms the push–pull character of these hemicyanine dyes derivatives with a significant intramolecular charge transfer from the functionalized donor group in the phenyl moiety to the methylbenzothiazolium acceptor group. The structure of compounds HDa and HDb has been identified by single crystal X-ray diffraction (see Fig. 1).
CH ethene protons at 7.86–8.24 ppm with coupling constants ≈15 Hz indicating their E conformation, and the N–CH2 protons of the benzothiazolium ring which resonate between 4.90 and 4.98 ppm. These data confirms the push–pull character of these hemicyanine dyes derivatives with a significant intramolecular charge transfer from the functionalized donor group in the phenyl moiety to the methylbenzothiazolium acceptor group. The structure of compounds HDa and HDb has been identified by single crystal X-ray diffraction (see Fig. 1).
Characterization of the layered hybrid material
Hemicyanine-modified montmorillonites (MMT–HDa–d) were prepared via cationic exchange procedure using the above-mentioned dyes. The reaction between HD+I− and Na–montmorillonite provided color solids. Once the organo-clays were prepared, the isolated solid materials have been characterized by a set of analysis including FTIR, XRD, TGA, solid state UV-vis/fluorescence and fluorescence microscopy. The IR spectra are used to highlight the presence of some characteristic vibration bands of functions specific to clays, as well as that of the organic matter by the appearance of different absorption bands corresponding to the cationic dye.The successful modification of MMT–Na has been evidenced by FTIR analysis. Fig. 2 shows example of the spectra of HDa, MMT–HDa and MMT–Na+. For the spectrum of the parent sodium–montmorillonite. Three bands absorption exist in the region 4000–1500 cm−1. The band at 3625 cm−1 is assigned to the stretching vibration of structural (octahedral) hydroxyl (O–H) groups linked either to Al3+ or Mg2+. The peak at 1635 cm−1 and the broad band at 3440 cm−1 were assigned to –OH bending and stretching vibration modes of adsorbed water respectively. The strong absorption band at 1035 is attributed to Si–O stretching vibrations. The FT-IR spectrum of the MMT–Na+ also shows bands in the 400–600 cm−1 area that are ascribed to Mg–O and Al–O bending vibration.43
In the spectra of HDa dye, the peaks for aliphatic and aromatic groups confirm the structure of the compound (Fig. 2c). When the corresponding dye was inserted into the MMT–Na layered the FT-IR spectra of the hybrid material show the bands of the MMT–Na+ as well as cationic dye with slight shift (Fig. 2b). Besides, the broad signal of the Si–O–Si at 1047 cm−1. Three bands were observed in the MMT–HDa at 1585, 1515 and 1443 cm−1 can be attributed to the stretching's vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N aromatic rings in the structure of dye. Whereas, a small absorption bands with low-intensity are corresponding to asymmetrical and symmetrical C–H stretching modes of methyl (CH3) and methylene (CH2) groups in the structure are unreadable due to the short-chain of ethyl group. These data, in sum, confirm the presence of the organic guest intercalation agent without altering its chemical structure.
N aromatic rings in the structure of dye. Whereas, a small absorption bands with low-intensity are corresponding to asymmetrical and symmetrical C–H stretching modes of methyl (CH3) and methylene (CH2) groups in the structure are unreadable due to the short-chain of ethyl group. These data, in sum, confirm the presence of the organic guest intercalation agent without altering its chemical structure.
Additional insight concerning the MMT intercalation by the cationic dyes has been gained by XRD analysis. This allows to evaluate different periodicities and particularly in our case, the d001 frequency and provides the exact value of the basal distance between lamellar MMT layers. In order to avoid the effect of the adsorbed water molecules in the interlamellar space, the samples used for this study were all dried at 80 °C for 1 night. The XRD patterns in the range of 2–10° are shown in Fig. 3 for the samples of MMT–Na+ with adsorbed dye. The clear distinction of MMT–Na+ and MMT dye complex is the diffraction peak corresponding to the (001) plane. Peak for MMT–Na+ was observed at 2θ = 7.26° while the diffraction peaks of MMT–HDa–d were found approximately at ≈5.45°. The comparison of MMT–Na+ with MMT dye complex shows a shifting of the reflection band to a shorter value. This shifting of the diffraction peaks of MMT dyes complexes to lower angle indicated that intercalations structures have been formed. This operation is named “organophilization of layered silicates” Scheme 3. If this process is incomplete, it is possible to find from the XRD pattern the interlayer distances of both the intercalated and original phases. In the present case, MMT dye gives rise to unique single phase with specific interlayer distance. As compared with that of pristine sodium–montmorillonite, the basal spacing increased from 1.17 nm to 1.63 nm due to the insertion/organization of dyes into the sheets of MMT as well as the amount of the exchanged organic dyes. Further, the obtained d-spacing value was direct proof of the negligible effect of the substituents in the phenyl ring of the corresponding dyes. According to Yariv44 a basal spacing of less than 1.4 nm does not permit any kind of aggregation of the dye cation to take place inside the interlayer space. The adsorbed dye cations probably form monolayers of monomeric species in the interlayers, with aromatic rings lying parallel to the silicate layer or almost parallel with the very small tilting. Parallel orientation should be facilitated by π interactions between the organic dyes and O-planes of the smectites. In this study, the interlayer expansion (gallery height) was obtained by subtracting the thickness of an individual layer of montmorillonite (0.96 nm) from the observed basal spacing; the gallery distance of the entire intercalated series was calculated to be 0.67 nm. Judging from the gallery height and the size of dyes, the orientation of the intercalated cation was discussed. The molecular length and breadth of four dyes determined from the software ChemBio3D after energy minimization were 1.28–1.49 nm and ≈0.65 nm, respectively.
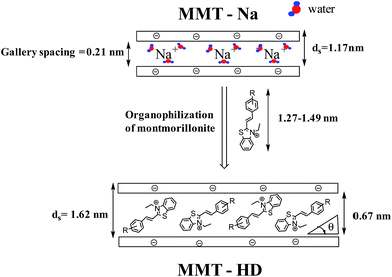 | ||
| Scheme 3 Representation of MMT–Na clay sheet, dye molecule (HDa–d) before and after organophilization process. | ||
As previously discussed, the gallery space was estimated to be about 0.67 nm. This value was lower than the vertical length and much higher than the thickness of the dye molecules. Therefore, the MMT–HD suggests an interdigited monolayer of the dye oriented flat with the molecular long axis inclined to the silicate layer by a tilte angle (θ < 54.7°).45–47 A flat arrangement of these molecules with conjugated D–π–A system within the silicate layers after dye modification was assumed. In fact, the dye can interact with the anionic sites of the clay surface at the terminal benzothiazolium nitrogen atom; this is called “head-on type” adsorption.48 The results for basal spacing of pure and the intercalated MMT are shown in Table 1.
| Samples | 2θ (°) | ds (nm) |
|---|---|---|
| MMT–Na | 7.26 | 1.17 |
| MMT–HDa | 5.45 | 1.63 |
| MMT–HDb | 5.43 | 1.62 |
| MMT–HDc | 5.45 | 1.63 |
| MMT–HDd | 5.44 | 1.62 |
The possible arrangement of cationic molecules in MMT layers has been suggested from XRD data in which their optical behavior would be strongly affected. These changes should get reflected in UV-vis and photoluminescence spectroscopy. Generally speaking, the physical attachment of dye to MMT affects its absorption or emission spectra. However, if the absorption or emission spectra of intercalates are shifted to higher wavelength (red-shifted band) as compared to that of pure dyes, there is J-type aggregation of dyes in the interlayer region, whereas H-type aggregation refers to the aggregation showing a blue-shifted band (hypsochromic band or H-band). The emission spectra will express the information about dye–dye as well as dye–clay interactions.49
Solid state UV-vis spectra of entire cationic compounds and their intercalated are presented in Fig. 4. Each dye has a specific wavelength around (423–482 nm). In the host–guest hybrid materials, the absorption band gets some red shifting to 480, 500, 445 and 477 nm in MMT–HDa, MMT–HDb, MMT–HDc and MMT–HDd, respectively. This red shifting of ≈16 nm is due to the J-type aggregation of hemicyanine dye units into the interlayer position of MMT.
To demonstrate the J-aggregation in MMT intercalated layer, we have studied the solid-state fluorescence spectra of the dye-intercalated MMT and pure dyes (Fig. 5). The dyes exhibit significant fluorescence with intense emission at room temperature when intercalated into clay. These significant red shifting to higher wavelength varied depending on the type of substituent on the phenyl ring. These results indicate strong π–π interaction between successive dye moieties and interactions of cationic dyes with MMT nanogalleries.50 Therefore, the MMT–HD clearly suggests J-type aggregation of dye into the interlayer position of MMT, which is also supported by UV-vis and XRD analysis.
The study of photophysical properties and orientation of dye molecules at clay mineral interfaces has only recently been clearly identified. Numerous research articles have been published in this respect and valuable results reported. As can be depicted from Table 2, both the orientation/arrangement and type-aggregation of dye into clay are strongly depending on many parameters. The structure and the amount of loaded dye as well as the interlayer distances of the dye/clay are among key factors which affect the photophysical properties of the guest–host hybrid materials.
| Dye–clay | Orientationa | Interlayer distances (nm) | Aggregation type | Photophysical properties of dye–clay | Ref. | ||
|---|---|---|---|---|---|---|---|
| λex | λem | ||||||
| a Depend on the amount of loaded dye and the interlayer distances of dye–clay.b Depend on the R substituted in the phenyl ring. | |||||||
| 1 | Perylenediimide–MMT | Interdigitated monolayer | 0.74–1.24 | J-Type | 482 | 675–695 | 51 |
| 2 | Azobenzene–MMT | Interdigitated monolayer or monolayer coverage | 0.37–0.9 | J-Type | 345–353 | — | 52 |
| 3 | Azobenzene–magadiite | Interdigitated monolayer or bilayer coverage | 1.57 | H-Type | 335–340 | — | 53 |
| 4 | Rhodamine B–MMT | — | — | J-Type | 250–371 | 420–622 | 54 |
| 5 | Cyanine–MMT | — | — | J-Type | 525 | — | 55 |
| 6 | Styrylbenzothiazolium–MMT | Interdigitated monolayer | 0.67 | J-Type | 445–500b | 519–575b | This work |
In order to have a deeper insight on the arrangement of the inorganic matrix in the nanocomposite materials, fluorescence confocal images have been recorded. Fig. 6 shows a series of fluorescence images recorded on dyes in a scan range where the size particles can be observed. These particles are due to the clay aggregates which are fluorescent (bright colour) and observable; the emission from the host–guest material is due to the cationic dye, which was intercalated in the surface of MMT–Na+ sheets. In addition, the particles present a homogeneous fluorescence distribution in terms of intensity and spectral properties since the particle features have all the same colours.
Thermogravimetric analysis was done to evaluate the thermal stability of the cationic dyes as well as for MMT dyes. TGA and DTG curves of different benzothiazolium dyes are shown in Fig. 7. The thermal studies reveal important information about the stability and thermal behavior of these compounds. Both decomposition temperatures (Tonset, Tmax) were determined by DTG and reported in Table 3. The thermogravimetric analysis and derivative curves show that the decomposition/degradation occurs in two steps. A first major weight loss was occurred between 220–400 °C (presented by two characteristic peaks) corresponded to the degradation first of the benzothiazolium salt moiety and then the styryl ring. The last degradation step between 450–730 °C can be represent the oxidation of the organic cation which results in the formation of H2O, CO2 and NO2.56 It can be noted that only one of the samples (HDd) exhibit a weight loss between 30 and 145 °C which may be due to the evaporation of residual solvents recrystallization or the presence of some bound moisture. In terms of thermal properties, the decomposition temperatures revealed that HDb had the highest thermal stability comparing to other dyes, starting to decompose at 259 °C. Therefore, the presence of hydroxyl group in the styryl ring efficiently enhanced its thermal stability. These results indicated that the thermal properties of these dyes varied depending on the types and the position of the substituents within the styryl ring.
| HD | R | Tonset.1 (°C) | Tmax.1 (°C) | Tonset.2a (°C) | Tmax.2b (°C) |
|---|---|---|---|---|---|
| a Tonset.2 refers to peak valley temperature between 1st peak and 2nd peak in DTG.b Tmax.2 refers to peak maximum temperature of 2nd DTG peak. Onset: decomposition starting temperature. | |||||
| HDa | 4-OCH3 | 219 | 241 | 248 | 289 |
| HDb | –OH | 259 | 271 | 281 | 329 |
| HDc | –Cl | 224 | 240 | 249 | 291 |
| HDd | 2-OCH3 | 214 | 230 | 242 | 304 |
Fig. 8 shows the TGA results performed on the entire intercalated MMT during heating under air. As can be seen from this figure all samples had low amount of moisture; they lost about ≈2% of water as moisture content, as can be seen by drop in its mass below 100 °C. While for the untreated MMT, the water uptake was approximately 6.62%. This initial step of weight loss is due to physically adsorbed water molecules. These results suggest a change from a hydrophilic to a hydrophobic nature due to the incorporation of the dye molecules. Similar results were obtained about the dehydration peaks in the case of azo dye intercalated MMT–Na+.57 Briefly, after the initial moisture, the Fig. 9 shows two major distinct regions of weight loss on heating. Organic dye substance evolves from 285 °C to 550 °C. Dehydroxylation of the aluminosilicate occurs from 600 °C to 700 °C, observed also in the parent MMT–Na. The content of intercalated hemicyanine dye could be determined by TGA. Thus, the styryl dye content was estimated to be 11.72 wt% of the intercalation complex. We can see that the thermal stability of the intercalated dye is significantly enhanced. The onset of weight loss for the intercalated dye did not occur until 290 °C, while that for the pure dye began at 219 °C. In other words, the onset of decomposition of MMT–HDa shifted to higher temperatures compared to pure HDa. The enhanced stability of cationic dye in MMT is attributed to limited mobility of the intercalated dye and the special confinement within the silicate galleries.58,59 Considering the results of XRD, FTIR analysis, it is implied that the intercalation of HD molecules has occurred in interlayer spaces of MMT–Na. Therefore, its thermal stability has improved accordingly.
 | ||
| Fig. 9 TGA of (a) MMT–Na, (b) MMT–HDa, and (c) HDa. The samples were heated to 800 at 10 °C min−1 in air atmosphere. | ||
Characterization of the bio-film
FTIR analysis attempted to characterize the effect of organoclay incorporation on the chitosan films and to determine the infrared bands and shifts related to MMT–HD/chitosan interactions. The position of the peaks of chitosan film spectrum is similar to those described by different authors. Fig. 10a show FT-IR spectrum of neat chitosan and, CS/MMT–HDa nanocomposites films at different weight percentage (1, 2, 5, 10 wt%). The absorption peaks of the pure chitosan films CS are mainly assignable to the stretching intra- and intermolecular O–H vibrations at 3500–3000 cm−1, overlapped with amine and secondary amide stretching mode. Symmetric and asymmetric C–H vibrations at 2920–2870 cm−1. Amide I vibrational mode (acetamido group) at 1635 cm−1, and amide II (amino group) at 1542 cm−1.60 Indeed, due to the incorporation of organo-modified montmorillonite to the chitosan matrix, some differences can be observed in the FTIR spectra of the bio-nanocomposites films. The intensity of the bands in the range of 1600–1300 cm−1 increases comparing to the bands of the pure chitosan. Interestingly, the band at 1026 cm−1 corresponding to asymmetric Si–O–Si stretching had its intensity increased after organoclay addition. It is also interesting to note that a modification resulted in stretching C–H vibrations at 2920–2870 cm−1, with an inverted intensity of methylene compared to methyl bands after treatment with organoclay (Fig. 10b). In fact, the introduction of MMT–HDa into the chitosan leads to an increase at 2877 cm−1 corresponding to methyl groups from methoxy branch of phenyl ring and a decrease at 2843 cm−1 related to methylene groups from alkyl chain of benzothiazolium dye. Further introduction of various kinds of modified clays into chitosan did not show much difference in light of a related chemical structure found in chitosan (NH and CH) and organoclays. Accordingly, there is a difficulty in identifying the interaction through the ammonium site on the modifier to interact with chitosan, although some literature observed widening of FTIR bands or multiplicity that may be attached to the electrostatics interactions between clay and other bio-based polymers such as soy protein or gelatin.61 | ||
| Fig. 10 FT-IR spectra of (a) chitosan and chitosan/MMT–HDa bio-nanocomposites and (b) enlargement in the 3100–2700 cm−1 frequency range. | ||
Contact angle is defined as the angle between the substrate surface and the tangent line at the point of contact of the liquid droplet with the substrate.62 The contact angle (CA) of water is one of the basic wetting properties of packaging materials and is an indicator of the hydrophilic/hydrophobic properties of the material. Usually, the more hydrophilic a material is, the lower the contact angle value it has. According to Araújo et al.63 angles greater than 50° indicate a hydrophobic surface. The surface hydrophobicity of the bio-nanocomposite film was evaluated by means of contact angle determination. Table 4 shows contact angles of bio-nanocomposite film, exhibiting a value of 85° for CS control film. Almeida and coworkers64 reported a contact angle of 95°, attributing this result to the hydrophobicity of chitosan chains. For the bio-nanocomposites the results indicate that the organoclay content addition affect film surface hydrophobicity, it can be clearly seen that an increase in organoclay content brought about an increase in contact angle values of the films. This observation can be explained by the fact that interaction between chitosan and modified montmorillonite reduced the availability of hydrophilic groups, thus decreasing the hydrophilicity. This interaction increased the distance between the clay layers, increasing hydrophobicity of films as the organoclay concentration increased. Furthermore, the effect of chain length corresponding to the hydrophobic groups is particularly important to interfacial properties and technologies of films. However, it should be noted that the slight increase of CA is due to the short chain containing two carbons of the dye. In this case, for CS/MMT–HDa, the contact angles of films increase with increase in the organoclay content from 1, 2, 5 and 10%, respectively. Considering all these aspects, the obtained results revealed that the wettability of the bio-nanocomposite films decreased with an increase in the organoclay content owing to the replacement of Na+ in the interlayer region of MMT with hydrophobic benzothiazolium dyes.
| MMT–HDa content (%) | CA (°) | WS (%) |
|---|---|---|
| a Each value is the mean of 3 replicates with the standard deviation; CA, contact angle of water; WS, water solubility. | ||
| Neat CS | 85.0 ± 0.5 | 12.3 ± 0.9 |
| Cs/MMT–HDa1% | 87.9 ± 1.5 | 10.1 ± 1.2 |
| Cs/MMT–HDa2% | 90.7 ± 1.6 | 9.8 ± 1.4 |
| Cs/MMT–HDa5% | 94.6 ± 0.8 | 9.2 ± 0.6 |
| Cs/MMT–HDa10% | 98.0 ± 1.7 | 8.7 ± 0.2 |
The water solubility of the bio-nanocomposite films as a function of MMT dye content is also shown in Table 4. Increasing organoclay concentration in film matrix caused to decrease in water solubility of the films. In the case of CS/MMT–HDa. The WS was 12.3% for the sample without clay and decreased to 10.1, 9.8 and 9.2% for the films containing 1, 2, 5 wt% MMT, respectively. Though, significant decreases in solubility observed in high levels of MMT. At the level of 10% wt% MMT, CS/MMT–HDa bio-nanocomposite showed the lowest WS values at 8.7%. It should be noted that the same behavior have been observed in all films CS/MMT–HDa–d comparing with the neat one. As a result, the reduction of the film solubility is indicative of a better interaction between chitosan chains and clay in the film, which is facilitated by a better dispersion of the nanoclay into the polymer matrix. Some authors observed similar behavior when MMT added to films composed of agar and soy-protein.65,66 In our case, the hydroxyl groups of organoclay can form strong hydrogen bonds with the hydroxyl groups on chitosan, thus improving the interactions between the molecules, improving the cohesiveness of biopolymer matrix and decreasing the water sensitivity. Solubility could be an important characteristic for biodegradable films because it can affect resistance of film to water. For example, films on high-moisture foods must be insoluble, while films for water-soluble pouches must be readily soluble.
The bio-films have been prepared and its fluorescence properties were analyzed at the optimum excitation wavelengths. As we know, chitosan is transparent in the UV-vis visible region and shows poor optical properties. Commercial chitosan has a very weak absorption and emission band on the contrary; the CS/MMT–HDa–d bio-nanocomposites films have shown excellent optical properties. All film exhibit an increase in the emission wavelengths with the increasing of the clay content starting from 1 to 10% (Fig. 11). In the same point of view, the intensity of fluorescence increases almost gradually. As stated before, there is an electrostatic attraction between the cationic dyes and negative charge on the clay particles. These interactions of the cationic dyes with the clay have several unique advantages in their photophysical properties such as restriction of molecular, internal-torsional motions, reduction of self-aggregation, increase of rigidity, reduction of strong excitation coupling, protection the fluorophore dye from the quenching action of dioxygen, and decrease of vibration relaxation pathways.67 In addition, the dispersion of photoactive or photoresponsive clays is generally performed to simply transfer the photophysical properties of the hybrid host–guest material to a polymer matrix.68 In our case, the incorporation of the organoclay into chitosan matrix enhance the fluorescence emission for the organoclays, suggesting the presence of both dye into the clay and dye dispersed in the chitosan matrix. To reach a direct visualization of the labeled clays into the polymer, the bio-nanocomposite materials have been investigated by fluorescence imaging through a confocal microscope, providing pictures with bright particles accounting the dispersion level of the platelets. The fluorescence images recorded on the bio-nanocomposite materials are shown in Fig. 12. The bio-film samples presented small fluorescent particles. These fluorescent particles are due to the clay aggregates labeled by the dye inside the chitosan matrix. This grain analysis indicates that the inorganic matrix is well dispersed in the polymer matrix with no space between clay and polymer. The fluorescence distribution is spectrally homogeneous since the bright areas in the image appear of same colors. This observation suggests that the hybrid host–guest materials are uniformly dispersed in the chitosan matrix with a small amount of agglomerates.
 | ||
| Fig. 11 Emission wavelengths of bio-films in terms of the clay content%; excitation wavelengths: Cs/MMT–HDa (λex = 412), Cs/MMT–HDb (λex = 435), Cs/MMT–HDc (λex = 395), Cs/MMT–HDd (λex = 410). | ||
Conclusions
Several hemicyanine dyes with donor–acceptor group have been successfully intercalated into the interlayer space of montmorillonite by the conventional ion exchange method, as indicated by FTIR spectra. As compared with that of pure dye, the thermal stability of the intercalated dye was greatly enhanced, as indicated by TGA curve, and the emission band corresponding to hemicyanine group in intercalated HD shifted towards longer wavelength. This could be ascribed to J-type aggregate HD in the interlayer space which exhibited the presence of an interdigited monolayer of dye into the host–guest material. The intercalation of the dye in the clay layers may find application in melt processing of thermoplastic polymers. In addition, the dried organophilic clays were then mixed with chitosan to obtain the corresponding bio-films. Both fluorescence and hygroscopic properties were reported. The results show enhancement in the hydrophobicity of the hybrid materials, whereas the fluorescent emission wavelength were also increase with increasing the clay content. According to confocal fluorescence image, the homogeneous fluorescence distribution suggests that between the filler and the polymer there is no space occupied by the dye. These results show an improvement of the interfacial adhesion and random dispersion/distribution of clay nano-particles which can afford a fluorescent bio-nanocomposite film with potential applications in packaging and biosensors.References
- A. Gandini, T. M. Lacerda, A. J. F. Carvalho, E. Trovatti, F. H. Isikgor and C. Remzi Becer, Polym. Chem., 2015, 6, 4497–4559 RSC.
- S. Grijalvo, J. Mayr, R. Eritja and D. D. Díaz, Biomater. Sci., 2016, 4, 555–574 RSC.
- M. K. Thakur, V. K. Thakur, R. K. Gupta and A. Pappu, ACS Sustainable Chem. Eng., 2016, 4, 1–17 CrossRef CAS.
- Y. Dou, X. Huang, B. Zhang, M. He, G. Yin and Y. Cui, RSC Adv., 2015, 5, 27168–27174 RSC.
- K. Y. Lee, Y. Aitomäki, L. A. Berglund, K. Oksman and A. Bismarck, Compos. Sci. Technol., 2014, 105, 15–27 CrossRef CAS.
- P. Bordes, E. Pollet and L. Avérous, Prog. Polym. Sci., 2009, 34, 125–155 CrossRef CAS.
- V. Siracusa, P. Rocculi, S. Romani and M. D. Rosa, Trends Food Sci. Technol., 2008, 19, 634–643 CrossRef CAS.
- L. A. M. Van Den Broek, R. J. I. Knoop, F. H. J. Kappen and C. G. Boeriu, Carbohydr. Polym., 2015, 116, 237–242 CrossRef CAS PubMed.
- V. Zargar, M. Asghari and A. Dashti, ChemBioEng Rev., 2015, 2, 204–226 CrossRef CAS.
- G. Geisberger, E. B. Gyenge, D. Hinger, A. Käch, C. Maake and G. R. Patzke, Biomacromolecules, 2013, 14, 1010–1017 CrossRef CAS PubMed.
- R. M. N. V Kumar, R. A. A. Muzzarelli, H. Sashiwa and A. J. Domb, Chem. Rev., 2004, 104, 6017–6084 CrossRef PubMed.
- E.-K. Lim, W. Sajomsang, Y. Choi, E. Jang, H. Lee, B. Kang, E. Kim, S. Haam, J.-S. Suh, S. J. Chung and Y.-M. Huh, Nanoscale Res. Lett., 2013, 8, 467 CrossRef PubMed.
- W. Thakhiew, M. Champahom, S. Devahastin and S. Soponronnarit, J. Food Eng., 2015, 158, 66–72 CrossRef CAS.
- L. Sánchez-González, C. González-Martínez, A. Chiralt and M. Cháfer, J. Food Eng., 2010, 98, 443–452 CrossRef.
- U. Siripatrawan and B. R. Harte, Food Hydrocolloids, 2010, 24, 770–775 CrossRef CAS.
- J. B. Marroquin, K. Y. Rhee and S. J. Park, Carbohydr. Polym., 2013, 92, 1783–1791 CrossRef CAS PubMed.
- A. Giannakas, K. Grigoriadi, A. Leontiou, N. M. Barkoula and A. Ladavos, Carbohydr. Polym., 2014, 108, 103–111 CrossRef CAS PubMed.
- K. Lewandowska, A. Sionkowska, B. Kaczmarek and G. Furtos, Int. J. Biol. Macromol., 2014, 65, 534–541 CrossRef CAS PubMed.
- S. Pandey and S. B. Mishra, J. Colloid Interface Sci., 2011, 361, 509–520 CrossRef CAS PubMed.
- E. Günister, D. Pestreli, C. H. Ünlü, O. Atici and N. Güngör, Carbohydr. Polym., 2007, 67, 358–365 CrossRef.
- M. Darder, M. Colilla and E. Ruiz-Hitzky, Chem. Mater., 2003, 15, 3774–3780 CrossRef CAS.
- S. Sahoo, A. Sasmal, D. Sahoo and P. Nayak, J. Appl. Polym. Sci., 2010, 118, 3167–3175 CrossRef CAS.
- J. Sun and Y. Jin, J. Mater. Chem. C, 2014, 38, 8000–8011 RSC.
- L. C. Schmidt, V. C. Edelsztein, C. C. Spagnuolo, P. H. Di Chenna and R. E. Galian, J. Mater. Chem. C, 2016, 29, 7035–7042 RSC.
- G. Chen, H. Qiu, P. N. Prasad and X. Chen, Chem. Rev., 2014, 114, 5161–5214 CrossRef CAS PubMed.
- K. Li, D. Ding, D. Huo, K. Y. Pu, N. N. P. Thao, Y. Hu, Z. Li and B. Liu, Adv. Funct. Mater., 2012, 22, 3107–3115 CrossRef CAS.
- X. Zhao, R. P. Bagwe and W. Tan, Adv. Mater., 2004, 16, 173–176 CrossRef CAS.
- I. Momiji, C. Yoza and K. Matsui, J. Phys. Chem. B, 2000, 104, 1552–1555 CrossRef CAS.
- H. Tomioka and T. Itoh, J. Chem. Soc., Chem. Commun., 1991, 532 RSC.
- D. Janeba, P. Capkova and Z. Weiss, J. Mol. Model., 1998, 4, 176–182 CrossRef CAS.
- M. Ogawa and K. Kuroda, Chem. Rev., 1995, 95, 399–438 CrossRef CAS.
- M. Ogawa, T. Ishii, N. Miyamoto and K. Kuroda, Appl. Clay Sci., 2003, 22, 179–185 CrossRef CAS.
- K. Ogawa, M. Wads and T. Kuroda, Langmuir, 1995, 11, 4598–4600 CrossRef.
- N. Kakegawa and M. Ogawa, Appl. Clay Sci., 2002, 22, 137–144 CrossRef CAS.
- Z. Klika, H. Weissmannová, P. Čapková and M. Pospíšil, J. Colloid Interface Sci., 2004, 275, 243–250 CrossRef CAS PubMed.
- D. W. Kim and A. Blumstein, Chem. Mater., 2001, 13, 243–246 CrossRef CAS.
- F. López Arbeloa and V. Martínez Martínez, Chem. Mater., 2006, 18, 1407–1416 CrossRef.
- R. N. Dsouza, U. Pischel and W. M. Nau, Chem. Rev., 2011, 111, 7941–7980 CrossRef CAS PubMed.
- Z. Chen, F. Li and C. Huang, Curr. Org. Chem., 2007, 11, 1241–1258 CrossRef CAS.
- K. Han, H. Li, X. Shen, G. Tang, Y. Chen and Z. Zhang, Comput. Theor. Chem., 2014, 1044, 24–28 CrossRef CAS.
- S.-Y. Gwon, B. A. Rao, H.-S. Kim, Y.-A. Son and S.-H. Kim, Spectrochim. Acta, Part A, 2015, 144, 226–234 CrossRef CAS PubMed.
- T. Deligeorgiev, A. Vasilev, S. Kaloyanova and J. J. Vaquero, Color. Technol., 2010, 126, 55–80 CAS.
- J. Madejová, Vib. Spectrosc., 2003, 31, 1–10 CrossRef.
- H. C. Shmuel Yariv, in Organo-Clay Complexes and Interactions, CRC Press, New York, 2001, pp. 463–566 Search PubMed.
- J. Sauer, M. Hofkens and J. Enderlein, in Handbook of Fluorescence Spectroscopy and Imaging: From Single Molecules to Ensembles, Wiley-VCH Verlag GmbH & Co. KGaA, 2011, pp. 1–30 Search PubMed.
- Y. Deng, W. Yuan, Z. Jia and G. Liu, J. Phys. Chem. B, 2014, 118, 14536–14545 CrossRef CAS PubMed.
- A. Eisfeld and J. S. Briggs, Chem. Phys., 2006, 324, 376–384 CrossRef CAS.
- R. Sasai, T. Shichi, K. Gekko and K. Takagi, Bull. Chem. Soc. Jpn., 2000, 73, 1925–1931 CrossRef CAS.
- G. Leone, U. Giovanella, W. Porzio, C. Botta and G. Ricci, J. Mater. Chem., 2011, 34, 12901–12909 RSC.
- F. Würthner, Z. Chen, F. J. M. Hoeben, P. Osswald, C. C. You, P. Jonkheijm, J. V. Herrikhuyzen, A. P. H. J. Schenning, P. P. A. M. Van Der Schoot, E. W. Meijer, E. H. A. Beckers, S. C. J. Meskers and R. A. J. Janssen, J. Am. Chem. Soc., 2004, 126, 10611–10618 CrossRef PubMed.
- C. Chakraborty, K. Dana and S. Malik, J. Phys. Chem. C, 2012, 116, 21116–21123 CAS.
- M. Ogawa, T. Ishii, N. Miyamoto and K. Kuroda, Appl. Clay Sci., 2003, 22, 179–185 CrossRef CAS.
- M. Ogawa, Society, 2002, 3304–3307 CAS.
- L. K. Joseph, H. Suja, G. Sanjay, S. Sugunan, V. P. N. Nampoori and P. Radhakrishnan, IOP Conf. Ser.: Mater. Sci. Eng., 2015, 73, 012040 CrossRef.
- N. Miyamoto, R. Kawai, K. Kuroda and M. Ogawa, Appl. Clay Sci., 2000, 16, 161–170 CrossRef CAS.
- A. Landau, A. Zaban, I. Lapides and S. Yariv, J. Therm. Anal. Calorim., 2002, 70, 103–113 CrossRef CAS.
- S. Raha, N. Quazi, I. Ivanov and S. Bhattacharya, Dyes Pigm., 2012, 93, 1512–1518 CrossRef CAS.
- S. Dultz and J. Bors, Appl. Clay Sci., 2000, 16, 15–29 CrossRef CAS.
- S. Ganguly, K. Dana, T. K. Mukhopadhyay and S. Ghatak, J. Therm. Anal. Calorim., 2011, 105, 199–209 CrossRef CAS.
- C. Paluszkiewicz, E. Stodolak, M. Hasik and M. Blazewicz, Spectrochim. Acta, Part A, 2011, 79, 784–788 CrossRef CAS PubMed.
- J. F. Martucci and R. a. Ruseckaite, Polym.-Plast. Technol. Eng., 2010, 49, 581–588 CrossRef CAS.
- S. Rivero, M. A. García and A. Pinotti, J. Agric. Food Chem., 2012, 60, 492–499 CrossRef CAS PubMed.
- E. A. Araujo, P. C. Bernardes, N. J. de Andrade, P. É. Fernandes and J. P. N. de SÁ, Aliment. Nutr., 2009, 20, 491–497 Search PubMed.
- E. V. R. Almeida, E. Frollini, A. Castellan and V. Coma, Carbohydr. Polym., 2010, 80, 655–664 CrossRef CAS.
- S.-I. Hong, H.-H. Lee and J.-W. Rhim, The 11th International Congress on Engineering and Food (ICEF), 2011 Search PubMed.
- I. Echeverría, P. Eisenberg and A. N. Mauri, J. Membr. Sci., 2014, 449, 15–26 CrossRef.
- J. Bujdák, Appl. Clay Sci., 2006, 34, 58–73 CrossRef.
- S. Coiai, E. Passaglia, A. Pucci and G. Ruggeri, Materials, 2015, 8, 3377–3427 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data and the relevant results. CCDC 1512675 and 1512676. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra23320a |
| This journal is © The Royal Society of Chemistry 2016 |










