Few molecule SERS detection using nanolens based plasmonic nanostructure: application to point mutation detection†
Abstract
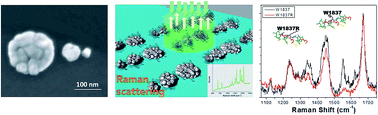
Advancements in nanotechnology fabrication techniques allow the possibility to design and fabricate a device with a minimum gap (<10 nm) between the composing nanostructures in order to obtain better control over the creation and spatial definition of plasmonic hot-spots. The present study is intended to show the fabrication of nanolens and their application to single/few molecules detection. Theoretical simulations were performed on different designs of real structures, including comparison of rough and smooth surfaces. Various molecules (rhodamine 6G, benzenethiol and BRCA1/BRCT peptides) were examined in this regard. Single molecule detection was possible for synthetic peptides, with a possible application in early detection of diseases.


 Please wait while we load your content...
Please wait while we load your content...