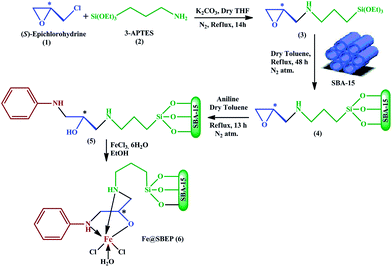
A new recyclable functionalized mesoporous SBA-15 catalyst grafted with chiral Fe(III) sites for the enantioselective aminolysis of racemic epoxides under solvent free conditions†
Mita Halderac,
Piyali Bhanjab,
Susmita Royc,
Swarbhanu Ghoshc,
Sudipta Kundub,
Md. Mominul Islamc and
Sk. Manirul Islam*c
aDepartment of Chemistry, University of Calcutta, 92 APC Road, Kolkata 700 009, India
bDepartment of Materials Science, Indian Association for the Cultivation of Science, 2A & B, Raja S. C. Mullick Road, Jadavpur, Kolkata-700032, India
cDepartment of Chemistry, University of Kalyani, Nadia, Kalyani, 741235, W.B., India. E-mail: manir65@rediffmail.com; Fax: +91-33-2582-8282; Tel: +91-33-2582-8750
First published on 5th October 2016
Abstract
An efficient and enantioselective strategy for the catalytic ring opening of racemic meso and terminal epoxides with aromatic as well as cyclic amine derivatives has been reported over a new mesoporous SBA-15 catalyst grafted with a chiral Fe(III) complex. The catalyst generates the expected chiral β-amino alcohols in over all better yields along with brilliant enantioselectivity (ee upto >99%) under solvent-free reaction condition at RT within 2–4 h reaction time. The catalyst is characterized in detail by PXRD, BET, HR-TEM, EPR, AAS, CHN analysis, IR and UV-Vis spectroscopic analysis etc. The catalyst is easily recoverable from the reaction system and can be used for five consecutive times without considerable loss of its catalytic activity as well as selectivity, which suggest sustainability of this chiral mesoporous catalyst.
Introduction
Day by day, the scope and utility of chiral technologies have gained very high impact. The synthesis of fine chemicals, drugs and non-linear optical materials cannot be possible without optically as well as enantiomerically pure molecules. Several approaches are available to generate chiral compounds, counting chemical derivatization, chiral resolution and asymmetric catalysis.1 Asymmetric catalytic transformations over chiral transition-metal catalysts are one of the most resourceful methods for obtaining optically pure products. Synthetic organic chemists show a prime interest to the transition metal catalysed asymmetric ring-opening (ARO) of epoxides using different types of amines because of its unprecedented activity to furnish various kinds of biologically and also commercially significant, enantiomerically pure β-amino alcohols.2 In this context, a number of homogeneous catalysts have been synthesized which can efficiently catalyze the ARO of terminal and meso-epoxides by aromatic as well as cyclic amines.3–5 However, most of these catalytic conditions have need of elevated temperature, long time, stoichiometric amounts of catalyst and hazardous solvent and furnish the product with low enantioselectivity. As a result, designing of stable, mild, low-priced, eco-friendly, and recyclable heterogeneous catalyst for the synthesis of enantiopure β-amino alcohols continue to attract the attention of researchers.In this context, due to the ease of product separation, easy recovery of the high-priced chiral catalysts, heterogeneous chiral catalytic systems have attracted a wide attention.6,7 Mesoporous materials have witnessed a remarkable growth in academic and industry because of their unusually huge surface area, tuneable pore size distribution, ease of functionalization with reactive organic groups/metal complexes and of course high hydrothermal as well as mechanical stability.8 As a consequence, synthesis of various mesoporous silica supported chiral metal complexes and their application in various organic transformations as an asymmetric heterogeneous catalyst have been an active area of research.9 However, the design and successful use of chiral mesoporous catalysts is still quite limited.10 Among various ordered mesoporous silica materials, SBA-15 with 2D-hexagonally ordered pore channels is considered an excellent support as it is convenient to functionalize, bearing high surface area and good thermal stability.11
Herein, we have synthesized a new chiral iron complex of (S)-amino alcohol-supported SBA-15 material Fe@SBEP via condensation of (S)-epichlorohydrine with aminopropyl trialkoxysilane followed by grafting this chiral organosilane species throughout the surface of SBA-15 material, then epoxide ring opening by aniline and finally complexation with Fe(III)-salt (Scheme 1).
Catalytic activity of Fe@SBEP has been explored in the field of ARO of meso and terminal epoxides with various aromatic as well as cyclic amines to produce highly regio- and enantio-selective β-amino alcohols in over all better yields (up to 98%) along with brilliant enantioselectivity (up to >99%) at RT in solvent free condition (Scheme 2). The mesoporous chiral catalyst has been recycled five repeating reaction cycles and it showed very good retention of product yields as well as enantioselectivity.
Results and discussion
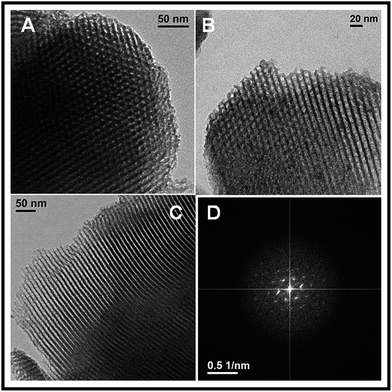
Characterization
Catalytic activity
To examine the catalytic activity of this catalyst, the first goal is to optimize the reaction condition, accompanied by reaction time, temperature, solvent and the catalyst amount. For this epoxide ring opening reaction, cyclohexene oxide and aniline as a nucleophilic partner, are used as model substrate to test the activity of Fe@SBEP and the results are shown in Table 1. Although, the ARO reactions were performed using different solvents like DCM, toluene, and H2O (Table 1, entries 2–4), the most superior result in term of yield and enantioselectivity has been observed under solvent-free condition. The reaction is quite sensitive to the temperature. It was quite evident from the table that with increase in temperature (40 °C) enantioselectivity of the product decreased (Table 1, entry 5), while below room temperature also caused the same (Table 1, entry 9). We have optimised the catalyst loading and reaction time (Table 1, entry 6–11). It is found that 0.5 mol% (15 mg) of catalyst loading provides best performance within 2 h (entry 8). In terms of yield (96%) and enantioselectivity (97%), the best result is obtained by performing the reaction without any solvent at RT for 2 h using only 15 mg (0.5 mol%) of the chiral Fe@SBEP catalyst (Table 1, entry 8). In the absence of catalyst, no product is obtained under these conditions (Table 1, entry 1).| Entry | Catalyst amt (mg) | Solvent | Temp. (°C) | Time | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|---|
| a Condition: cyclohexene oxide (1 mmol), aniline (1 mmol), Fe@SBEP (15 mg, 0.5 mol%), without solvent, rt.b Isolated yield.c Determined by HPLC using Chiralpak OD-H column. | ||||||
| 1 | — | — | 40 | 24 | — | nd |
| 2 | 20 | DCM | 40 | 4 | 80 | 71 |
| 3 | 20 | Toluene | 40 | 4 | 65 | 69 |
| 4 | 20 | H2O | 40 | 4 | 85 | 81 |
| 5 | 20 | — | 40 | 4 | 94 | 82 |
| 6 | 20 | — | rt | 4 | 96 | 95 |
| 7 | 15 | — | rt | 3 | 96 | 97 |
| 8 | 15 | — | rt | 2 | 96 | 97 |
| 9 | 15 | — | 10 | 2 | 78 | 93 |
| 10 | 10 | — | rt | 2 | 85 | 97 |
| 11 | 15 | — | rt | 1 | 81 | 94 |
After optimizing the reaction condition, we examined other substrates with Fe@SBEP catalyst for the ARO reaction of different kinds of meso and terminal epoxides alike cyclohexene oxide (1a), allyl glycidyl ether (1b), 1,2-epoxy-3-phenoxy propane (1c), epichlorohydrine (1d), propylene oxide (1e) styrene oxide (1f) and glycidyl isopropyl ether (1g) with aniline (2a), substituted anilines [4-methoxy aniline (2b), 3-chloro aniline (2c), 2-iodo aniline (2d)] and cyclic amines [pipyridine (2d), morpholine (2e)] (Table 2). Irrespective of electron withdrawing or donating group attached with phenyl ring of the aniline derivatives all substrates gave the asymmetric amino alcohols as the final products with excellent conversion (87–98%) along with high enantioselectivity (72 to >99%) within 2–4 h reaction time. The less hindered carbon atom of the epoxide ring of allyl glycidyl ether was attacked by aniline under regioselective manner to form the respective product with moderate enantioselectivity (ee, 72%) having S configuration (Table 2, entry 1). Styrene oxide also under goes regioselective cleavage by aniline to furnish S-configured β-amino alcohol (Table 2, entry 10). Except allyl glycidyl ether and styrene oxide, all of the listed terminal epoxides gave the analogous amino alcohols having R configuration with good regio- and enantioselectivity. It is quite evident from Table 2, that all of the terminal epoxides gave only one single regioisomer without any traces of bis-adduct. Glycidyl isopropyl ether reacts with aniline to give the corresponding product (Table 2, entry 15) with good yield and enantioselectivity. Meso epoxide like cyclohexene oxide gave the corresponding (1R,2R)-amino alcohols with different substituted anilines with high yield (yield 90–96%) and enantioselectivity (ee, 98–99%) (Table 2, entry 5–7). Pipyridine (2d), morpholine (2e) react with cyclohexene oxide efficiently to give the corresponding amino alcohols in good yield (86–88%) and enantioselectivity (76–82%) (Table 2, entry 8 and 9). While, aliphatic amines like n-propyl amine and n-butyl amine gave no traces of product in the similar condition (Table 2, entry 10 and 11).
| Entry | Epoxide | Amine | Time (h) | Productb | Yieldc (%) | eed (%) | TOF (h−1) |
|---|---|---|---|---|---|---|---|
| a Conditions: epoxide (1 mmol), amine (1 mmol), Fe@SBEP (15 mg, 0.5 mol%), without solvent, RT.b Absolute configurations were determined by comparing with the literature reports.c Isolated yield.d Determined by HPLC using Chiralpak OD-H column. | |||||||
| 1 | 1b | 2a | 2.5 |  |
93 | 72 | 74 |
| 2 | 1c | 2a | 2 | 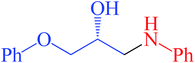 |
98 | 93 | 98 |
| 3 | 1d | 2a | 2 |  |
95 | >99 | 95 |
| 4 | 1e | 2b | 3 |  |
87 | 88 | 58 |
| 5 | 1a | 2a | 2 |  |
96 | 97 | 96 |
| 6 | 1a | 2b | 4 | 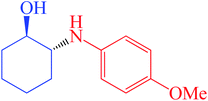 |
95 | >99 | 47 |
| 7 | 1a | 2c | 3 |  |
90 | >99 | 60 |
| 8 | 1a | 2d | 3.5 |  |
88 | 82 | 50 |
| 9 | 1a | 2e | 3.5 |  |
86 | 76 | 49 |
| 10 | 1f | 2a | 2 |  |
95 | 98 | 95 |
| 11 | 1a | n-Propyl amine | 8 | — | — | — | — |
| 12 | 1a | n-Butyl amine | 8 | — | — | — | — |
| 13 | 1e | 2a | 2 |  |
97 | 89 | 97 |
| 14 | 1b | 2d | 3 |  |
87 | 78 | 58 |
| 15 | 1g | 2a | 3 |  |
88 | 95 | 59 |
To explain the efficiency and applicability of our present catalytic system, we have summarized the comparative results with reference to the literature reports in Table S1.† From this table, it is obvious that our Fe@SBEP catalyst provides a better activity and enantioselectivity for the corresponding ARO reaction over the reported systems.
Recycling of catalyst
Ease of separation, recoverability and reusability are the most important part for a heterogeneous catalyst. The reusability of the Fe@SBEP catalyst was inspected for the representative ARO reaction between 1,2-epoxy-3-phenoxy propane and aniline. After the completion of the reaction, the entire part was centrifuged to collect the solid catalyst from the mixture. It was then washed thoroughly and repetitively with double distilled water followed by DCM and finally dried in oven at 90 °C for 6 h for reuse. Fig. 5 efficiently indicates that the catalyst can be recycled and reused for five times successfully without appreciable diminish in product yield and enantioselectivity. This fact is confirmed by the EPR spectrum of the reused catalyst which indicates that the oxidation state of iron centres in the catalyst remains unchanged after recycle (Fig. 6).Conclusion
We have synthesized chiral Fe@SBEP catalyst and its catalytic activity was examined in the field of enantioselective asymmetric ring opening (ARO) of different kinds of meso- and terminal epoxides with various aromatic as well as cyclic amines. Generally, the expected chiral β-amino alcohols are formed in good to very good yields with excellent regio- and enantioselectivity (generally 72 to >99% ee) without any solvent and within short reaction time at RT. The mesoporous chiral catalyst has 2D-hexagonally ordered porous structure, high BET surface area and good thermal stability. Further, low catalyst loading, environmentally benign reaction pathway and high recycling efficiency make the process green and cost effective. Thus we believe that this protocol for designing and successful use of chiral catalyst will find a useful application in green synthesis of enantiopure chiral β-amino alcohols.Experimental section
Experimental procedure for synthesis of Fe@SBEP catalyst
The synthetic protocol of the chiral ligand (5) and the catalyst Fe@SBEP (6) were out lined in the Scheme 1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) for 10 h. Finally the sample was dried under vacuum at 45 °C.
30) for 10 h. Finally the sample was dried under vacuum at 45 °C.Acknowledgements
SMI acknowledges Department of Science and Technology, (DST-SERB, Project No. SB/S1/PC-107/2012 dated 10.06.2013), New Delhi, Govt of India, University Grant Commission (UGC, Project F. No. 43-180/2014(SR) dated 25th July 2015), New Delhi, Govt of India and Department of Science and Technology, Govt of West Bengal (WB-DST, Project Sanction F. No. 811(sanc.)/ST/P/S & T/4G-8/2014, Dt-04/01/2016) for financial support. M. H. and M. M. I. acknowledges UGC for their fellowships. S. R. acknowledges Kalyani University for providing her fellowship (URS). We gratefully acknowledge DST and UGC, New Delhi, Govt of India for providing fund to the Dept of Chemistry, University of Kalyani through PURSE, FIST and SAP programme. SMI also acknowledges University of Kalyani for providing personal research assistance grant (PRG). We are thankful to Prof. Asim Bhaumik, Department of Materials Science, IACS, Kolkata for providing necessary characterization facility for this research work.References
- (a) H. Nishiyama and J. Ito, Chem. Commun., 2010, 4, 203 RSC; (b) J.-P. Genet, T. Ayad and V. Ratovelomanana-Vidal, Chem. Rev., 2014, 114, 2824 CrossRef CAS PubMed.
- (a) S. Hashiguchi, A. Kawada and H. Natsugari, J. Chem. Soc., Perkin Trans. 1, 1991, 2435 RSC; (b) S. Knapp, Chem. Rev., 1995, 95, 1859 CrossRef CAS; (c) S. Horri, H. Fukase, T. Matsuo, Y. Kameda, N. Asano and K. Matsui, J. Med. Chem., 1986, 29, 1038 CrossRef; (d) M. Pastor and M. Yus, Curr. Org. Chem., 2005, 9, 1 CrossRef; (e) D. J. Ager, I. Prakash and D. R. Schaad, Chem. Rev., 1996, 96, 835 CrossRef CAS PubMed; (f) A. K. Ghosh, G. Bilcer and G. Schiltz, Synthesis, 2001, 15, 2203 CrossRef.
- (a) X.-L. Hou, J. Wu, L.-X. Dai, L.-J. Xia and M.-H. Tang, Tetrahedron: Asymmetry, 1998, 9, 1747 CrossRef CAS; (b) S. Sagawa, H. Abe, Y. Hase and T. Inaba, J. Org. Chem., 1999, 64, 4962 CrossRef CAS PubMed; (c) G. Bartoli, M. Bosco, A. Carlone, M. Locatelli, M. Massaccesi, P. Melchiorre and L. Sambri, Org. Lett., 2004, 6, 2173 CrossRef CAS PubMed.
- (a) F. Carrée, R. Gil and J. Collin, Org. Lett., 2005, 7, 1023 CrossRef PubMed; (b) C. Ogawa, S. Azoulay and S. Kobayashi, Heterocycles, 2005, 66, 201 CrossRef CAS; (c) K. Arai, M. M. Salter, Y. Yamashita and S. Kobayashi, Angew. Chem., Int. Ed., 2007, 46, 955 CrossRef CAS PubMed; (d) E. Mai and C. Schneider, Chem.–Eur. J., 2007, 13, 2729 CrossRef CAS PubMed; (e) S. Azoulay, K. Manabe and S. Kobayashi, Org. Lett., 2005, 7, 4593 CrossRef CAS PubMed.
- (a) K. Arai, S. Lucarini, M. M. Salter, K. Ohta, Y. Yamashita and S. Kobayashi, J. Am. Chem. Soc., 2007, 129, 8103 CrossRef CAS PubMed; (b) E. Mai and C. Schneider, Synlett, 2007, 13, 2136 Search PubMed; (c) H. Bao, J. Zhou, Z. Wang, Y. Guo, T. You and K. Ding, J. Am. Chem. Soc., 2008, 130, 10116 CrossRef CAS PubMed; (d) B. Gao, Y. Wen, Z. Yang, X. Huang, X. Liu and X. Feng, Adv. Synth. Catal., 2008, 350, 385 CrossRef CAS; (e) H. Bao, J. Wu, H. Li, Z. Wang, T. You and K. Ding, Eur. J. Org. Chem., 2010, 6722 CrossRef CAS; (f) M. Martin, A. El Hellani, J. Yang, J. Collin and S. Bezzenine-Lafollée, J. Org. Chem., 2011, 76, 9801 CrossRef CAS PubMed.
- R. I. Kureshy, I. Ahmad, N. H. Khan, S. H. R. Abdi, K. Pathak and R. V. Jasra, J. Catal., 2006, 238, 134 CrossRef CAS.
- S. Roy, P. Bhanja, S. S. Islam, A. Bhaumik and S. M. Islam, Chem. Commun., 2016, 52, 1871 RSC.
- (a) S. Roy, T. Chatterjee, B. Banerjee, N. Salam, A. Bhaumik and S. M. Islam, RSC Adv., 2014, 4, 46075 RSC; (b) B. Karimi, A. Zamani, S. Abedi and J. H. Clark, Green Chem., 2009, 11, 109 RSC; (c) M. H. Tucker, A. J. Crisci, B. N. Wigington, N. Phadke, R. Alamillo, J. Zhang, S. L. Scott and J. A. Dumesic, ACS Catal., 2012, 2, 1865 CrossRef CAS; (d) M. Pramanik and A. Bhaumik, ChemCatChem, 2014, 6, 2577 CrossRef CAS; (e) N. Vila, E. Andre, R. Ciganda, J. Ruiz, D. Astruc and A. Walcarius, Chem. Mater., 2016, 28, 2511 CrossRef CAS.
- (a) C. Chen, L. Y. Kong, T. Y. Cheng, R. H. Jin and G. H. Liu, Chem. Commun., 2014, 50, 10891 RSC; (b) X. M. Xu, T. Y. Cheng, X. C. Liu, J. Y. Xu, R. H. Jin and G. H. Liu, ACS Catal., 2014, 4, 2137 CrossRef CAS; (c) E. Verde-Sesto, M. Pintado-Sierra, A. Corma, E. M. Maya, J. G. de la Campa, M. Iglesias and F. Sanchez, Chem.–Eur. J., 2014, 20, 5111 CrossRef CAS PubMed; (d) T. Kawasaki, Y. Araki, K. Hatase, K. Suzuki, A. Matsumoto, T. Yokoi, Y. Kubota, T. Tatsumi and K. Soai, Chem. Commun., 2015, 51, 8742 RSC; (e) A. J. McCue, R. P. K. Wells and J. A. Anderson, ChemCatChem, 2011, 3, 699 CrossRef CAS; (f) L. L. Lou, K. Yu, F. Ding, X. Peng, M. Dong, C. Zhanga and S. Liu, J. Catal., 2007, 249, 102 CrossRef CAS; (g) R. I. Kureshy, I. Ahmad, N. H. Khan, S. H. R. Abdi, K. Pathak and R. V. Jasra, Tetrahedron: Asymmetry, 2005, 16, 3562 CrossRef CAS; (h) K. Yu, Z. Gu, R. Ji, L. L. Lou, F. Ding, C. Zhang and S. Liu, J. Catal., 2007, 252, 312 CrossRef CAS.
- (a) V. J. Mayani, S. H. R. Abdi, R. I. Kureshy, N. H. Khan, S. Agrawal and R. V. Jasra, J. Chromatogr. A, 2006, 1135, 186 CrossRef CAS PubMed; (b) J. A. Kelly, M. Giese, K. E. Shopsowitz, W. Y. Hamad and M. J. MacLachlan, Acc. Chem. Res., 2014, 47, 1088 CrossRef CAS PubMed; (c) D. C. Zhang, J. Y. Xu, Q. K. Zhao, T. Y. Cheng and G. H. Liu, ChemCatChem, 2014, 6, 2998 CrossRef CAS; (d) T. Y. Cheng, Q. K. Zhao, D. C. Zhang and G. H. Liu, Green Chem., 2015, 17, 2100 RSC; (e) M. Mandal, V. Nagaraju, B. Sarma, G. V. Karunakar and K. K. Bania, ChemPlusChem, 2015, 80, 749 CrossRef CAS; (f) V. J. Mayani, S. H. R. Abdi, R. I. Kureshy, N. H. Khan, S. Agrawal and R. V. Jasra, J. Chromatogr. A, 2008, 1191, 223 CrossRef CAS PubMed; (g) R. A. Garcia, R. van Grieken, J. Iglesias, V. Morales and D. Gordillo, Chem. Mater., 2008, 20, 2864 Search PubMed; (h) P. Dutta, P. Kalita and P. K. Baruah, ChemistrySelect, 2016, 1, 1650 CrossRef CAS.
- (a) D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS PubMed; (b) P. F. Fulvio, S. Pikus and M. Jaroniec, J. Mater. Chem., 2005, 15, 5049 RSC.
- S. K. Kundu, J. Mondal and A. Bhaumik, Dalton Trans., 2013, 42, 10515 RSC.
- H. P. Chun, T. Weyhermüller, E. Bill and K. Wieghardt, Angew. Chem., Int. Ed., 2001, 40, 2489 CrossRef CAS.
- S. K. Das, M. K. Bhunia and A. Bhaumik, J. Solid State Chem., 2010, 183, 1326 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra23289b |
| This journal is © The Royal Society of Chemistry 2016 |