Fabrication of antimicrobial polyethersulfone microfiltration membranes by corona plasma-assisted coating of silver nanoparticles†
Samaneh Afkham,
Abdolreza Aroujalian* and
Ahmadreza Raisi
Department of Chemical Engineering, Amirkabir University of Technology (Tehran Polytechnic), Tehran, Iran. E-mail: aroujali@aut.ac.ir
First published on 8th November 2016
Abstract
In this work, antimicrobial polyethersulfone (PES) membranes were fabricated by coupling of corona treatment and coating silver nanoparticles for use in the microfiltration of milk in order to reduce its microbial content. For this purpose, the microfiltration membranes were prepared by vapor-induced phase inversion coupled with a nonsolvent-induced phase inversion technique and the surface of the membranes were modified by corona air plasma under various operating conditions. Finally, the silver nanoparticles were coated on the surface of the corona treated PES membrane via a dip coating technique. The separation performance, antimicrobial properties and biofouling resistance of the silver coated membranes were evaluated in the microfiltration of skim milk. The results indicated that the exposure time and applied power of the corona treatment had significant effects on the surface modification of the PES membranes and affected the antimicrobial and anti-biofouling activities of the prepared membranes. Also, the amount of silver coated on the membrane surface and the silver ion released during the microfiltration process depended on the corona treatment conditions. The silver coated membranes showed high antimicrobial activity which resulted in anti-biofouling properties and higher separation performance than the neat PES microfiltration membrane.
Introduction
Microfiltration (MF) is a low pressure-driven membrane separation process which has found particular applications in the dairy industry due to its mild operation conditions. The microfiltration process is widely employed in the dairy industry for the microbial reduction from milk, fat removal from milk and whey as well as separation of micellar casein from whey protein in skim milk to increase the cheese making process yield.1 Generally, ceramic membranes have been used for the microfiltration of the dairy products by various researchers.2–4 For example, Elwell and Barbano3 used a ceramic membrane with a pore size of 1.4 μm for the microbial reduction of skim milk and observed that at a certain storage temperatures, the shelf life of the microfiltrated skim milk was more than commercially pasteurized milk. Beolchini et al.4 applied a tubular ceramic membrane to reduce the microbial content of bovine and ovine milk. Despite the desirable mechanical properties and economical processing capabilities, the polymeric microfiltration membranes have been rarely employed in the dairy industry. There are only a few researches on the protein removal from milk by the microfiltration process with the polymeric membranes.5–7 For instance, Lawrence et al.6 and Beckman et al.7 used polyvinylidene fluoride spiral-wound membrane for micellar casein concentrate production.Membrane fouling and biofouling are the major problems in the application of microfiltration process and other membrane processes such as ultrafiltration and nanofiltration in the dairy industry. The biofouling is the result of undesirable deposition and accumulation of microorganisms on the membrane surface. The membrane biofouling results in a decrease in the membrane flux, deterioration of the membrane structure and an increase in energy and membrane replacement costs.8 Techniques such as chemical cleaning,9 addition of antimicrobial materials into the feed stream of the process,10 UV and ozonation treatment11 as well as application of membranes with antimicrobial properties12–15 have been previously applied to reduce the membrane biofouling. The most commonly used method is the addition of antimicrobial materials like chlorine into the feed stream because of the easy-handling process and cost effectiveness. However, due to stricter legislative regulation on the use of the chemical agents especially in the food products, other effective and environmental-friendly alternative is needed. Therefore, current researches focus on the membrane modification by incorporation of an antibacterial agent into the membrane that is well-known as antimicrobial membrane.
An antimicrobial membrane enables to kill microorganisms and inhibit microbial growth and as a result, biofilm formation can be substantially hindered and biofouling can be obstructed.16 For fabrication of the antimicrobial membranes, various components such as silver,12,17–22 gold,23 titanium dioxide,24 selenium,25 chlorinated compounds26 and enzymes27 have been used as antimicrobial agents. Among them, silver nanoparticles due to their superior electrical, stability and antibacterial properties and long-term activity against a broad spectrum of viruses and microorganisms have been extensively employed for the preparation of antimicrobial membranes. In the recent years, a number of studies have been conducted in exploring silver-incorporation to polymeric materials such as polysulfone (PSf),12 polyacrylonitrile (PAN),17 chitosan,18 polyethersulfone (PES),19–22 cellulose acetate (CA),28 polyvinylidene fluoride (PVDF),29 polyimide (PI)30 and polyamide (PA)31 for application in different membrane processes like microfiltration, ultrafiltration and reverse osmosis. The antimicrobial membranes containing silver nanoparticles could be prepared by incorporating the antimicrobial agent into the membrane matrix via blending the nanoparticles into the casting solution12,17,19–22 or by coating the antimicrobial agent on the membrane surface by immersing the membrane into the nanoparticles solution.22,30,31 Toroghi et al.22 prepared the silver nanoparticles/PES membranes by both blending and coating methods for using in the ultrafiltration of raw milk. They reported that the solution blending technique could be easily implemented but limits the loading of silver nanoparticles and alter the membrane morphology during the phase inversion process but, uniform distribution of the silver nanoparticles and controlled released antimicrobial membranes would be achieved via the coating approach without any change in the membrane morphology and performance. Recently, Sile-Yuksel et al.32 fabricated antimicrobial membranes by incorporation of silver nanoparticles into the PSf, PES and CA polymers by solution blending technique and observed that the membranes stored silver nanoparticles at surface had the best antibacterial properties.
In the coating technique, the interaction between the membrane surface and antimicrobial agent has a significant effect on the antimicrobial activity and separation performance of the obtained membranes. Commonly, the polymeric materials have chemically inert surfaces with low surface tensions. Therefore, the membrane surface modification before the coating process meliorates the interaction between the silver nanoparticles and the membrane surface and leads to enhance the membrane surface hydrophilicity and consequently increases the coating nanoparticles on the membrane surface. In previous studies, irradiation33 and plasma techniques such as corona discharge,34–37 glow discharge38 and radio frequency39 have been applied for the membrane surface modification. The corona discharge has received the most focus because of its advantages such as operating at atmospheric pressure, online process capability, high efficiency, scalability to a larger area and cost effectiveness.37 Sadeghi et al.34 investigated the corona treatment time and power effect on modifying PES ultrafiltration membrane surface to minimize the membrane fouling and improve the membrane separation performance. Sadeghnejad et al.36 employed corona air plasma to modify the surface of polyethylene film in order to develop silver coated packaging films with antibacterial properties. Their results showed that the surface modification enhanced the hydrophilic functional groups on the film surface which could bond with the silver nanoparticles. Recently, Moghimifar et al.35 applied the corona air plasma discharge to prepare TiO2 nanoparticles coated PES ultrafiltration membrane with antifouling properties.
The objective of this work is to fabricate the antimicrobial polyethersulfone microfiltration membrane with anti-biofouling property for using in the pasteurization of the skim milk. For this purpose, the PES microfiltration membranes were prepared by the vapour-induced phase inversion followed by nonsolvent-induced phase inversion technique and the membrane surface was modified by the corona air plasma at different treatment times and input powers. Then, the corona treated membranes were immersed in a uniform and colloidal silver nanoparticles solution prepared by the chemical reduction method. Finally, the antibacterial and antifouling properties and the separation performance of the prepared membranes were evaluated by the microfiltration of the skim milk. The main innovative aspect of this study is the preparation of antimicrobial microfiltration membranes by the combination of the corona air plasma treatment and coating of silver nanoparticles for microfiltration of milk as an alternative for the thermal pasteurization process. Another important contribution is the investigation of influence of the corona treatment conditions on the separation performance and antifouling properties of the modified membranes.
Experimental
Material
The commercial PES with molecular weight of 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 (Ultrason E 6020P) purchased from BASF (Ludwigshafen, Germany) was utilized as membrane material. N-Methyl-2-pyrrolidone (NMP) as solvent and triethyleneglycol (TEG) as nonsolvent additive supplied from Merck Co. Ltd. (Darmstadt, Germany) were used for membrane preparation. Analytical grade silver nitrate (AgNO3) and fructose as reactants and diammonium hydrogen citrate ((NH4)2HC6H5O7) as stabilizer received from Merck Co. Ltd. (Darmstadt, Germany) were used for silver nanoparticles preparation. Aqueous sodium hydroxide (NaOH) was used for pH adjustment. Potassium oxalate and formaldehyde with high purity utilized for the protein measurement were supplied from Merck Co. Ltd. (Darmstadt, Germany). Also, Muller Hinton agar used in the microbial tests was purchased from Merck Co. Ltd. (Darmstadt, Germany). Skim milk for the microfiltration experiments was supplied from Mimas Dairy Co. (Tehran, Iran).
000 g mol−1 (Ultrason E 6020P) purchased from BASF (Ludwigshafen, Germany) was utilized as membrane material. N-Methyl-2-pyrrolidone (NMP) as solvent and triethyleneglycol (TEG) as nonsolvent additive supplied from Merck Co. Ltd. (Darmstadt, Germany) were used for membrane preparation. Analytical grade silver nitrate (AgNO3) and fructose as reactants and diammonium hydrogen citrate ((NH4)2HC6H5O7) as stabilizer received from Merck Co. Ltd. (Darmstadt, Germany) were used for silver nanoparticles preparation. Aqueous sodium hydroxide (NaOH) was used for pH adjustment. Potassium oxalate and formaldehyde with high purity utilized for the protein measurement were supplied from Merck Co. Ltd. (Darmstadt, Germany). Also, Muller Hinton agar used in the microbial tests was purchased from Merck Co. Ltd. (Darmstadt, Germany). Skim milk for the microfiltration experiments was supplied from Mimas Dairy Co. (Tehran, Iran).
Preparation of PES membrane
The PES microfiltration membranes were fabricated by vapour-induced phase inversion coupled with nonsolvent-induced phase inversion technique.40 First, the PES polymer was dried at 100 °C for at least 4 h. Then the PES (10% wt) was solved in a mixture of NMP (40% wt) as the solvent and TEG (50% wt) as the nonsolvent additive. The homogenous polymer solution was de-aerated by a vacuum process for 2 h and cast on a glass plate with a steel casting knife. The glass plate was subjected to the humid air (relative humidity of 65–75%) for 1 min and then immersed into a bath containing de-ionized water as non-solvent at 50 °C to complete the participation. After coagulation, the formed PES membrane was separated from the glass plate. The prepared membranes were stored in a soaking bath containing de-ionized water for 24 h for the complete removal of the residual solvent, and then were dried at the room temperature. The final thickness of the membranes was 80 ± 5 μm.Preparation of silver nanoparticles
The colloidal aqueous solution of silver nanoparticles was prepared via the chemical reduction of silver nitrate using fructose as reducing agent and diammonium hydrogen citrate as stabilizer based on the method presented by Dehnavi et al.41 In this method, 1 g of fructose and 0.1 g of diammonium hydrogen citrate were dissolved in 1000 ml of de-ionized water and the pH of the solution was adjusted to 9.5 by a 1 M sodium hydroxide solution. The solution was stirred continuously and heated until the temperature reached 85 °C and remained constant at this temperature. Then, 9.35 ml of 0.1 M AgNO3 solution was added to the heated solution. After sometime, the solution turned to yellow indicating the formation of silver nanoparticles.Membrane surface modification
The prepared PES membranes were modified by the corona air plasma using a commercial device (Naaj Corona, Naaj Plastic Co., Tehran, Iran) at the atmospheric pressure according to the procedure presented by Moghimifar et al.35 For this purpose, the membrane samples with the size of 7 cm × 9 cm were placed on the backing roller covered with the silicon coating, rotating at a given speed. The distance between the backing roller and the aluminum electrode was adjusted to a specific value. The corona plasma was generated within the air gap between the electrode and backing roller. The membrane was treated when the generated corona came into contact with the membrane surface. The corona treatment was performed at various input powers (300, 500 and 700 W) for different time durations (7, 10 and 13 min).In order to coat the silver nanoparticles on the surface of PES membrane, the corona treated membranes attached on a glass plate were vertically immersed in the colloidal solution of silver nanoparticles at 85 °C for 60 min. The silver colloidal solution was stirred continually during the coating. Finally, the coated membranes were rinsed twice with the de-ionized water and dried at the room temperature for 24 h. The nomenclature of the prepared membranes is presented in Table 1.
| Membrane sample | Corona input power (W) | Corona time (min) | Silver coated |
|---|---|---|---|
| M1 | — | — | — |
| M2 | 300 | 7 | — |
| M3 | 300 | 10 | — |
| M4 | 300 | 13 | — |
| M5 | 500 | 7 | — |
| M6 | 500 | 10 | — |
| M7 | 500 | 13 | — |
| M8 | 700 | 7 | — |
| M9 | 700 | 10 | — |
| M10 | 700 | 13 | — |
| M11 | 300 | 7 | √ |
| M12 | 300 | 10 | √ |
| M13 | 300 | 13 | √ |
| M14 | 500 | 7 | √ |
| M15 | 500 | 10 | √ |
| M16 | 500 | 13 | √ |
| M17 | 700 | 7 | √ |
| M18 | 700 | 10 | √ |
| M19 | 700 | 13 | √ |
Characterization tests
The absorption spectrum of the silver nanoparticles solution was measured by a UV-Vis spectrophotometer (Jasco V550, JASC Co., Tokyo, Japan) at a wavelength range of 300–700 nm. The X-ray powder diffraction (XRD) pattern was recorded using a Philips instrument (X'pert diffractometer, Eindhovan, Netherlands) using CuKα radiation with a scanning speed of 0.03 (2θ) min−1 to determine the crystallinity of silver nanoparticles and the average crystal dimension was calculated using Scherrer's equation. Transmission electron micrograph (TEM) analysis of the silver nanoparticles was performed using a ZEISS EM-900 electron microscope (Carl Zeiss SMT, Peabody, NY, USA) operating at 50 kV. Also, dynamic light scattering (DLS) was performed using a Malvern ZetasizerNano (Malvern Instruments Ltd., Worcestershire, England) to determine the size distribution and hydrodynamic diameter of the silver nanoparticles.Fourier transmission infrared-attenuated total reflection (FTIR-ATR) spectroscopy was used to analyze the functional groups on the membranes surface. The FTIR-ATR instrument consisted of a Nicolet Nexus 670 spectrometer (Nicolet Instrument Co., Madison, WI, USA) with 4 cm−1 resolution over a wave number range of 1900–700 cm−1. For each test, a 1 cm × 4 cm sample without further treatment was used and the corona treated membranes were tested right after being exposed to the corona. Scanning electron microscopy (SEM) using a Hitachi SEM (model S-4160, Hitachi, NJ, USA) was applied to determine the morphology of the membrane surface and cross section as well as to detect the coated silver nanoparticles on the membrane surface. For the cross section images, the hydrate membrane samples were fractured by immersing them into the liquid nitrogen. Then all virgin and treated membranes were stuck on a sample holder and the samples were coated with a thin layer of gold layer by a sputtering system before the analysis. Atomic force microscopy (AFM) analysis was used to measure the changes of the membrane surface roughness before and after the membrane modification. The AFM tests were performed using a microscope (Nano Educator, NTMDT Co., Zelenograd, Russia) with a spatial resolution of approximately 2 nm in z direction. The instrument was calibrated by the standard samples (TGG1 and TGX1, NT-MDT Co., Zelenograd, Russia). The membrane samples were fixed on a holder double-sided tape and 10 mbr× 10 μm areas were scanned by semi-contact mode in the air. Three different locations were tested and the average values of roughness were reported. The roughness was expressed as RMS (root mean square) and RA (average roughness) values. An optical contact angle measurement system (OCA-20; Data Physics GmbH, Filderstadt, Germany) was also used to measure the water contact angle of the treated and untreated membranes surface at the room temperature with the de-ionized water. The contact angle of five different locations on the membrane surface was measured and the average values were reported as the contact angle for a membrane sample.
Furthermore, to determine the porosity of the prepared membrane, a dry membrane sample with a definite size was immersed in the de-ionized water bath for 24 h. The soaked membrane was picked up from bath and water on the surface of the membranes was carefully cleaned with a clean tissue and the membrane was weighed and set down as the membrane weight in the wet state (Ww). Then the membrane was dried for 24 h in a desiccator and weighed again to measure the membrane weight in dry state (Wd). The membrane porosity (ε) was calculated as follows:
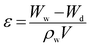 | (1) |
Finally, the mean pore diameter (Dp) of the membranes was estimated via the filtration velocity method using Guerout–Elford–Ferry equation.40
 | (2) |
![[small script l]](https://www.rsc.org/images/entities/char_e146.gif) is the membrane thickness (m), Q is the volume of the permeate water per unit time (m3 s−1), A is the membrane effective area (m2) and ΔP is the operating pressure (MPa).
is the membrane thickness (m), Q is the volume of the permeate water per unit time (m3 s−1), A is the membrane effective area (m2) and ΔP is the operating pressure (MPa).
Microfiltration of skim milk
The microfiltration performance of the prepared membranes was measured by a cross flow flat sheet membrane module with a filtration area of 35 cm2 at the room temperature and an operating pressure of 1.5 bar. The filtration apparatus has been previously described.22 Before the microfiltration process, the membrane setup was sterilized by a cleaning in place method. The untreated and silver coated membranes were tested with skim milk as feed stream. The permeate flux (J) was measured by collecting the permeate stream in the determined intervals and calculated using the following equation:
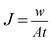 | (3) |
The protein permeability (Pp) of the membranes was determined by measuring the protein content of the feed and permeate streams. The protein permeability was calculated as follows:
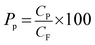 | (4) |
| Protein (%) = 1.74(V2 − V1) | (5) |
The silver content of the coated membrane as well as the silver release from the membranes during the microfiltration process were determined by the atomic absorption spectrophotometry (AAS) analysis using a Varian spectrophotometer (Varian SpectrAA-300, Varian, CA, USA). To measure the amount of coated silver nanoparticles on the various coated membranes, 2 cm × 2 cm samples of the coated membranes were immersed in 5 ml nitric acid (65% (v/v)) for 24 h. After digestion, the solution was diluted with 5 ml de-ionized water and then analyzed by AAS.
Finally, the microbial content of the feed, permeate and retentate streams was determined after 2 h filtration by the colony count and agar diffusion methods. In the first method, samples from the raw milk and permeate and retentate streams were diluted by saline solution (0.9% wt NaCl aqueous solution) with the proper ratio and spread uniformly on the Muller Hinton solid agar. The plates were incubated at 37 °C for 72 h, and then colonies formed on the nutrient agar were counted. The numbers of colonies multiplied by the dilution give the concentration of bacteria cells in the samples. In the agar diffusion tests, the agar was poured into the sterilized Petri dishes and was allowed to solidify. 100 μl of the milk samples was spread uniformly over the plates. Then the samples of the silver coated membranes were cut into the circular disks with a diameter of 2 cm and after the sterilization, they were put on the agar. The plates were incubated at 37 °C for 24 h. The clear zone formed around the samples was recorded by digital camera as an indication of inhibition of the microbial species. The neat membrane was used as the control sample.
Results and discussion
Characterization of the silver nanoparticles
Formation of silver nanoparticles by the proposed method was confirmed by the UV-Vis, XRD, TEM and DLS analysis and the results are presented in Fig. 1. The UV-Vis absorption spectrum of the silver nanoparticles solution is shown in Fig. 1a. It can be seen that the absorption spectrum has a single symmetric peak with a maximum absorption at wavelength of 425 nm. The shape and location of the UV-Vis spectrum peak depend on the size of silver particle and the concentration and particle size distribution of the silver particles depend on the height and width of the absorption peak. For the silver particles smaller than 100 nm, the UV-Vis absorption peak is located between 400 and 450 nm.44 Also, the value of full width at half maximum (FWHM) for the UV-Vis peak of silver nanoparticles was 90 nm. Therefore, the spectrum of the synthesized silver particles colloidal solution reveals that uniform silver particles in the nano form with a narrow size distribution were prepared by the chemical reduction method. | ||
| Fig. 1 The UV-Vis spectrum (a), XRD pattern (b), TEM image (c) and particle size distribution (d) of the silver nanoparticles. | ||
The XRD pattern of the prepared silver nanoparticles is indicated in Fig. 1b. The observed XRD pattern matches very well with the standard XRD powder pattern and shows the sharp peaks at 2θ of 38.3°, 44.5°, 64.8° and 77.7° assigned to the silver crystals. Using Scherrer's equation, the average crystal size of the nanoparticles was found to be 35 nm. Fig. 1c shows the TEM image of the silver nanoparticles. It can be observed that the spherical silver nanoparticles with very narrow size distribution were well-formed and well-dispersed.
Finally, the size distribution of the silver nanoparticles which was determined by the DLS analysis is shown in Fig. 1d. As indicated in this figure, the synthesized silver nanoparticles have narrow size distribution with an average diameter of 40 nm.
Characterization of the membranes
A particular change in the membrane surface polarity after modification by the corona treatment and subsequent coating of silver nanoparticles can be related to the changes in the functional groups on the membrane surface. The FTIR-ATR analysis was employed to evaluate the changes in the functional groups of the membrane surface. Fig. 2 illustrates the FTIR-ATR absorbance spectra of the treated and neat PES membrane. In these spectra, the peaks at wave numbers of 1100, 1240, 1150, 1320, 1480 and 1580 cm−1 can be attributed to the C–O, C–O–C, asymmetric and symmetric stretches of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and aromatic C
O and aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C asymmetric stretching vibration bonds of the PES membranes. The absorbance values of these functional groups for different membranes are given in Table S1 (see ESI†).
C asymmetric stretching vibration bonds of the PES membranes. The absorbance values of these functional groups for different membranes are given in Table S1 (see ESI†).
A comparison between the neat and corona treated membranes reveals that the absorbance of the C–O, C–O–C and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups increases after the corona treatment, while the absorbance of the C
O groups increases after the corona treatment, while the absorbance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups reduces. Also, the effect of corona treatment conditions, i.e. exposure time and input power, on the surface modification and functional group changes can be obviously observed from Fig. 2 and Table S1 (see ESI†). The results indicate that the required time for the groups build up decreases by increasing the corona power. As discussed by Sadeghi et al.34 and Moghimifar et al.35 the effect of corona treatment conditions on the surface modification can be related to the building effect and damaging effect during the corona treatment. These possible phenomena may counteract one another or overtake the other; therefore depending on the power and time of the corona treatment, different effects can be seen at different stages. Moreover, a comparison between the FTIR-ATR spectra of the corona modified membranes and the silver coated membranes indicate that the absorbance values of the functional groups slightly decreased by coating the silver nanoparticles on the membrane surface. This is due to the deposition of silver nanoparticles on the surface of the membrane and involving some of the functional groups with the silver particles. M5 hydrophilic functional groups absorption was in the highest level.
C groups reduces. Also, the effect of corona treatment conditions, i.e. exposure time and input power, on the surface modification and functional group changes can be obviously observed from Fig. 2 and Table S1 (see ESI†). The results indicate that the required time for the groups build up decreases by increasing the corona power. As discussed by Sadeghi et al.34 and Moghimifar et al.35 the effect of corona treatment conditions on the surface modification can be related to the building effect and damaging effect during the corona treatment. These possible phenomena may counteract one another or overtake the other; therefore depending on the power and time of the corona treatment, different effects can be seen at different stages. Moreover, a comparison between the FTIR-ATR spectra of the corona modified membranes and the silver coated membranes indicate that the absorbance values of the functional groups slightly decreased by coating the silver nanoparticles on the membrane surface. This is due to the deposition of silver nanoparticles on the surface of the membrane and involving some of the functional groups with the silver particles. M5 hydrophilic functional groups absorption was in the highest level.
The SEM analysis was used to evaluate the morphology and structure of the prepared PES membranes. Fig. 3 shows SEM image from the surface and cross section of the neat PES membrane. This image indicates that the PES membrane prepared by vapour-induced phase inversion coupled with nonsolvent-induced phase inversion method had skinless and sponge-liked structure. This pore structure is due to the delayed demixing during the membrane preparation. Similar structure was reported for the PES membrane by other researchers.40–45 For example, Shin et al.45 observed the sponge structure for the PES microfiltration membrane at high concentration of the 2-methoxy ethanol as nonsolvent additive in the casting solution. The SEM images from the surface of the silver coated PES membranes are presented in Fig. 4. A comparison between Fig. 3a and 4a to e illustrates that the silver nanoparticles were successfully coated on the surface of the corona modified PES membranes.
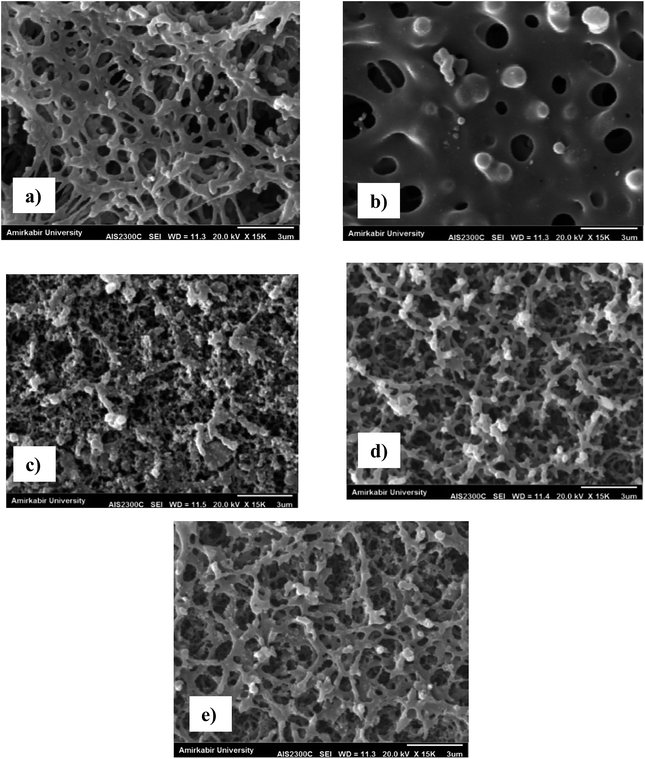 | ||
| Fig. 4 The SEM images from the surface of the silver coated membranes: (a) M11, (b) M12, (c) M13, (d) M15 and (e) M18. | ||
Furthermore, the AFM analysis was employed to characterize the changes in the roughness and topography of the membrane surfaces after coating the silver nanoparticles. The AFM images of the neat PES membrane and the silver coated membranes are presented in Fig. S1 (see ESI†). The Ra and RMS values of various membranes are also given in Table 2. It can be seen that the roughness parameters of all coated membranes were lower than the uncoated membrane. This indicates that coating silver nanoparticles on the corona modified membranes smoothes the membrane surface due to deposition of silver nanoparticles into the pores on the surface of the PES membranes.
| Membrane | RA (nm) | RMS (nm) |
|---|---|---|
| M1 | 507 | 383 |
| M11 | 291 | 334 |
| M12 | 296 | 350 |
| M13 | 226 | 351 |
| M14 | 110 | 142 |
| M15 | 231 | 295 |
| M16 | 286 | 328 |
| M17 | 142 | 259 |
| M18 | 178 | 213 |
| M19 | 190 | 220 |
The contact angle analysis was also employed to investigate the membrane hydrophilicity after the coating process. The values of contact angle of the neat and silver coated PES membranes are shown in Table 4. The contact angle results show that coating silver nanoparticles increases the membrane hydrophilicity. These results are in good agreement with the FTIR-ATR results. Similar observations were reported by Sadeghnejad et al.36 as the coated silver nanoparticles on the surface of polyethylene packaging films which treated by the corona air plasma.
Finally, the porosity and average pores size of the neat PES membrane and coated membranes are given in Table 3. It can be seen that the porosity of the silver coated membranes was closed to the porosity of the neat membrane and this reveals that coating silver nanoparticles on the membrane surfaces had no significant effect on the membrane porosities. However, the pore size values of the coated membranes differ from the average pore size of the neat PES membrane as shown in Table 3. This difference may be attributed to the corona modification of the membranes before the coating process as well as the deposition of silver nanoparticles into the membrane pores. The previous researches indicated that the corona treatment of the PES membranes changed the membrane pore size depending on the corona exposure time and applied power.34,35 For example, Moghimifar et al.35 observed that the untreated PES ultrafiltration membrane had lower average pore size than the modified ones and they related it to the corona damaging effect of the corona treatment which disrupted the upper structure of the membranes leading to a more open framework. On the other hand, the corona modification at higher exposure time or applied power is coupled with higher uptake of the silver nanoparticles on the membrane. Thus the possibility of silver deposition into the membrane pores enhances and leads to a decrease in the average pore size of the coated membranes.
| Membrane | Contact angle (°) | Average pore size (nm) | Porosity |
|---|---|---|---|
| M1 | 60 | 302 | 0.86 |
| M11 | 40 | 264 | 0.87 |
| M12 | 39 | 320 | 0.86 |
| M13 | 42 | 234 | 0.86 |
| M14 | 37 | 228 | 0.88 |
| M15 | 42 | 206 | 0.86 |
| M16 | 39 | 268 | 0.86 |
| M17 | 47 | 306 | 0.85 |
| M18 | 51 | 208 | 0.89 |
| M19 | 46 | 284 | 0.87 |
Microfiltration and antifouling performance of the membranes
In order to investigate the separation and antifouling performance of the prepared PES membranes, the microfiltration experiments using pure water and skim milk as feed stream were conducted. The pure water and milk permeation fluxes of different microfiltration membranes versus time are indicated in Fig. 5 and 6. In addition, the steady state water and milk fluxes as well as the protein permeability of the fabricated membrane are presented in Table 5. It can be observed that the pure water flux of the most of the silver coated membranes was lower than that of the neat PES membrane. This is due to the reduction in the pore size of the coated microfiltration membranes by the deposition of the silver nanoparticles into the membrane pores. On the other words, the milk flux of all coated microfiltration membranes was higher than the milk flux of the neat membrane. This is attributed to the anti-biofouling property of the silver coated membranes. Silver ion release from the coated membrane deactivates the microorganisms in the feed stream and consequently accumulation of the microorganisms on the membrane surface is decreased and results in lower biofouling phenomenon. The protein permeability values (Table 4) indicated that the M12 membrane sample had the highest protein permeability which can be related to higher anti-biofouling effect of silver nanoparticles for this membrane. Therefore, more milk and protein can be transferred through the membrane because of the lower mass transport resistance.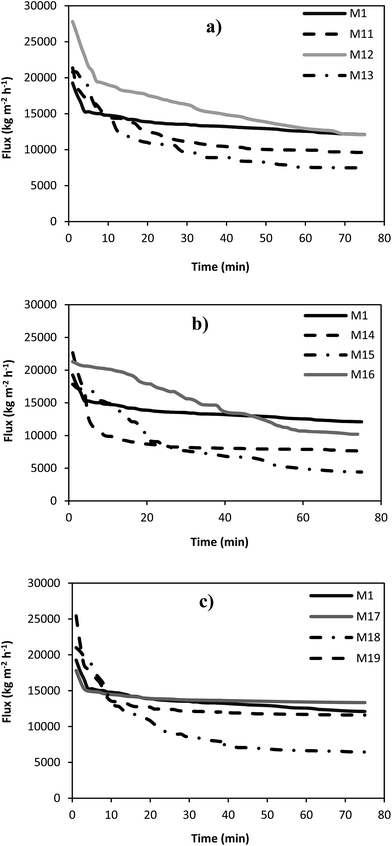 | ||
| Fig. 5 The pure water flux of the neat PES membrane and the silver coated membranes for various corona treatment times and input power of: (a) 300 W, (b) 500 W and (c) 700 W. | ||
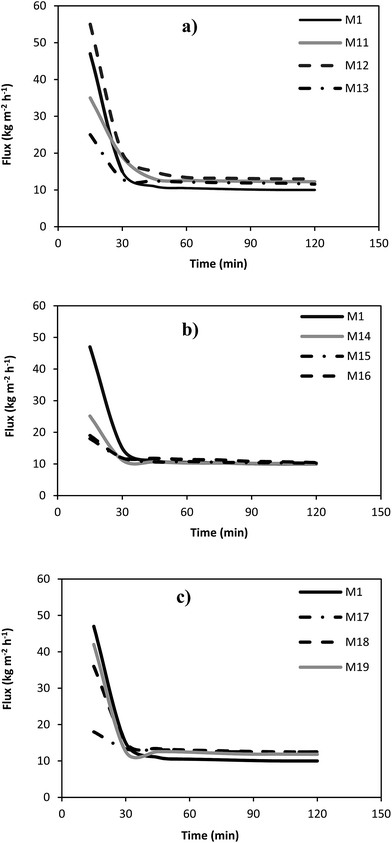 | ||
| Fig. 6 The milk flux of the neat PES membrane and the silver coated membranes for various corona treatment times and input power of: (a) 300 W, (b) 500 W and (c) 700 W. | ||
| Membrane | Water flux (kg m−2 h−1) | Milk flux (kg m−2 h−1) | Protein permeability (%) |
|---|---|---|---|
| M1 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
10 | 30 |
| M11 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
12.5 | 27 |
| M12 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
13.8 | 44 |
| M13 | 7500 | 11.8 | 23 |
| M14 | 8000 | 10.15 | 21 |
| M15 | 6500 | 11.8 | 18 |
| M16 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
10.8 | 25 |
| M17 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
12.5 | 38 |
| M18 | 7000 | 12.2 | 19 |
| M19 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
12 | 27 |
Moreover, the silver content of the coated membranes and silver ion release from the membrane in the permeate and retentate streams during the microfiltration of the pure water were determined by the AAS analysis and the results are given in Table 5. For all coated membranes, the silver release in the retentate is more than the permeate samples, since the retentate stream is in a direct contact with the membrane surface and more silver will release in the retentate stream. It can be observed from Table 5 that the M14 membrane sample had the highest silver content and the silver release from the M12 membrane in both permeate and retentate streams was higher than the other membranes. These observations are in consistent with the FTIR-ATR and AFM results. Based on the FTIR-ATR analysis, the polar functional groups like C–O, C–O–C and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O on the membrane treated by corona air plasma at input power of 500 W and 7 min exposure time were the highest between other membrane samples. Therefore, more silver nanoparticles were coated on the surface of the M14 membrane because of its hydrophilic functional groups after the corona treatment. However, the amount of silver released in water for the M12 membrane is higher than the others. Based on the AFM results, the roughness of the PES microfiltration membrane increased after corona treatment at input power of 300 W and exposure time of 10 min and the possibility of coating silver nanoparticles on the membrane surface because of van der Waals force between the silver and the membrane enhances, so silver will release more easily. For the M14 membrane, the FTIR-ATR results indicated that its hydrophilic functional group was more than the others and consequently silver nanoparticles interact covalently with the functional groups of the membrane surface. In this kind of interaction, the bonds are strong and silver doesn't release easily.
O on the membrane treated by corona air plasma at input power of 500 W and 7 min exposure time were the highest between other membrane samples. Therefore, more silver nanoparticles were coated on the surface of the M14 membrane because of its hydrophilic functional groups after the corona treatment. However, the amount of silver released in water for the M12 membrane is higher than the others. Based on the AFM results, the roughness of the PES microfiltration membrane increased after corona treatment at input power of 300 W and exposure time of 10 min and the possibility of coating silver nanoparticles on the membrane surface because of van der Waals force between the silver and the membrane enhances, so silver will release more easily. For the M14 membrane, the FTIR-ATR results indicated that its hydrophilic functional group was more than the others and consequently silver nanoparticles interact covalently with the functional groups of the membrane surface. In this kind of interaction, the bonds are strong and silver doesn't release easily.
| Membrane | Silver content (ppm cm−2) | Released silver in permeate (μg h−1) | Released silver in retentate (μg h−1) |
|---|---|---|---|
| M11 | 0.426 | 2.28 | 4.10 |
| M12 | 0.546 | 4.10 | 11.94 |
| M13 | 0.229 | 3.07 | 3.33 |
| M14 | 0.726 | 3.59 | 9.10 |
| M15 | 0.266 | 3.07 | 3.20 |
| M16 | 0.512 | 1.92 | 2.28 |
| M17 | 0.213 | 1.92 | 2.43 |
| M18 | 0.201 | 1.79 | 2.18 |
| M19 | 0.261 | 1.41 | 1.45 |
Finally, the antimicrobial properties of the prepared microfiltration were evaluated by colony count and agar diffusion methods and the results are presented in Fig. 7 and S2 (see ESI†), respectively. As shown in Fig. 7, the microbial content of the permeate and retentate samples of the silver coated membranes decreased significantly in comparison with the neat PES microfiltration membrane. This is due to the release of silver ions from the coated membrane into both permeate and retentate streams. Silver ions attract to the microorganisms and deactivate them. Therefore, the silver coated microfiltration membranes had significant antimicrobial performance on the permeate and retentate streams. The antimicrobial activity of the coated membranes are in good agreement with the results of silver ion release measurement in the permeate and retentate streams. Moreover, Fig. S2 (see ESI†) presents the results of agar diffusion test against E. coli and S. aureus for the neat and silver coated membranes. As can be seen, the silver coated membranes exhibit an antimicrobial performance and a clear zone can be seen around the coated membranes while it can't be observed around the neat membrane. This microbial inhibition around the coated membranes shows the release of silver from these membranes and its diffusion into the agar layer, which prevents the growth of microbial colonies around the membrane.
Conclusion
The PES microfiltration membranes with antibacterial and anti-biofouling properties were prepared by corona plasma-assisted coating silver nanoparticles. In this regard, the microfiltration membranes were fabricated by combination of the vapor-induced phase inversion and nonsolvent-induced phase inversion technique, modified by the corona air plasma and coated by silver nanoparticles via a dip coating process. The fabricated antibacterial membranes were employed for the microfiltration of skim milk in order to decrease the microbial load and increase the shelf life. The silver nanoparticles which were synthesized by the chemical reduction method were successfully coated on the surface of the corona treated membranes. The results indicated that the exposure time and applied power of the corona treatment had significant influence on the physical and chemical actions of the corona and affected the antibacterial and anti-biofouling of the obtained membranes. The AAS analysis results revealed that the amount of silver coated on the membrane surface as well as the amount of silver released during the microfiltration process depended on the corona treatment conditions. Based on the FTIR-ATR analysis, the polar functional groups on the membrane treated by corona air plasma at input power of 500 W and exposure time of 7 min were the highest between other membrane samples and more silver nanoparticles were coated on the surface of the M14 membrane because of its hydrophilic functional groups after the corona treatment. However, the amount of silver release from the M12 membrane was higher than the others. The silver coated PES membranes showed high antimicrobial and anti-biofouling activity against the milk microorganisms and led to a higher separation performance in comparison to the neat PES microfiltration membrane. Finally, it can be concluded that the antimicrobial microfiltration membranes could be fabricated by the corona plasma-assisted dip coating technique with no significant changes on the morphology and structure of the membrane and the obtained membranes can be used in the food and dairy industries.References
- L. V. Saboyainsta and J. L. Maubois, Lait., 2000, 80, 541–553 Search PubMed
.
- I. Pafylias, M. Cheryan, M. Mehaia and N. Saglam, Food Res. Int., 1996, 29, 141–146 CrossRef CAS
.
- M. Elwell and D. Barbano, J. Dairy Sci., 2006, 89, E20–E30 CrossRef PubMed
.
- F. Beolchini, A. Vegho and D. Barba, Desalination, 2004, 161, 251–258 CrossRef CAS
.
- J. Zulewska, M. Newbold and D. Barbano, J. Dairy Sci., 2009, 92, 1361–1377 CrossRef CAS PubMed
.
- N. Lawrence, S. Kentish, A. O'Connor, A. Barber and G. Stevens, Sep. Purif. Technol., 2008, 60, 237–244 CrossRef CAS
.
- S. Beckman, J. Zulewska, M. Newbold and D. Barbano, J. Dairy Sci., 2010, 93, 4506–4517 CrossRef CAS PubMed
.
- V. Kochkodan and N. Hilal, Desalination, 2015, 356, 187–207 CrossRef CAS
.
- S. L. Khor, D. D. Sun, Y. Liu and J. O. Leckie, Process Biochem., 2007, 42, 1641–1648 CrossRef CAS
.
- J. Yu, Y. Baek, H. Yoon and J. Yoon, J. Membr. Sci., 2013, 427, 30–36 CrossRef CAS
.
- C. Marconnet, A. Houari, D. Seyer, M. Djafer, G. Coriton, V. Heim and P. Di Martino, Desalination, 2011, 276, 75–81 CrossRef CAS
.
- K. Zodrow, L. Brunet, S. Mahendra, D. Li, A. Zhang, Q. Li and P. J. Alvarez, Water Res., 2009, 43, 715–723 CrossRef CAS PubMed
.
- H. Karkhanechi, F. Razi, I. Sawada, R. Takagi, Y. Ohmukai and H. Matsuyama, Sep. Purif. Technol., 2013, 105, 106–113 CrossRef CAS
.
- L. Y. Ng, A. W. Mohammad, C. P. Leo and N. Hilal, Desalination, 2013, 308, 15–33 CrossRef CAS
.
- V. Kochkodan, D. J. Johnson and N. Hilal, Adv. Colloid Interface Sci., 2014, 116–140 CrossRef CAS PubMed
.
- Y. Kim, D. Rana, T. Matsuura and W. J. Chung, Chem. Commun., 2012, 48, 693–695 RSC
.
- D. G. Yu, M. Y. Teng, W. L. Chou and M. C. Yang, J. Membr. Sci., 2003, 255, 115–123 CrossRef
.
- X. Zhu, R. Bai, K. H. Wee, C. Liu and S. L. Tang, J. Membr. Sci., 2010, 363, 278–286 CrossRef CAS
.
- H. Basri, A. F. Ismail and M. Aziz, Desalination, 2011, 273, 72–80 CrossRef CAS
.
- I. Sawada, R. Fachrul, T. Ito, Y. Ohmukai, T. Maruyama and H. Matsuyama, J. Membr. Sci., 2012, 387, 1–6 CrossRef
.
- H. Basri, A. F. Ismail and M. Aziz, Desalination, 2012, 287, 71–77 CrossRef CAS
.
- M. Toroghi, A. Raisi and A. Aroujalian, Polym. Adv. Technol., 2014, 25, 711–722 CrossRef CAS
.
- K. Balasubramanian, Colloids Surf., A, 2014, 455, 174–178 CrossRef
.
- V. Vatanpour, S. S. Madaeni, R. Moradian, S. Zinadini and B. Astinchap, Sep. Purif. Technol., 2012, 90, 69–82 CrossRef CAS
.
- D. Low, A. Hamood, T. Reid, T. Mosley, P. Tran, L. Song and A. Morse, J. Membr. Sci., 2011, 378, 171–178 CrossRef CAS
.
- K. Tan and S. K. Obendorf, J. Membr. Sci., 2007, 305, 287–298 CrossRef CAS
.
- S. Kroll, L. Treccani, K. Rezwan and G. Grathwohl, J. Membr. Sci., 2010, 365, 447–455 CrossRef CAS
.
- W. L. Chou, D. G. Yu and M. C. Yang, Polym. Adv. Technol., 2005, 16, 600–607 CrossRef CAS
.
- B. De Gusseme, T. Hennebel, E. Christiaens, H. Saveyn, K. Verbeken, J. P. Fitts, N. Boon and W. Verstraete, Water Res., 2011, 45, 1856–1864 CrossRef CAS PubMed
.
- Y. Deng, G. Dang, H. Zhou, X. Rao and C. Chen, Mater. Lett., 2008, 62, 1143–1146 CrossRef CAS
.
- S. Y. Lee, H. J. Kim, R. Patel, S. J. Im, J. H. Kim and B. R. Min, Polym. Adv. Technol., 2007, 18, 562–568 CrossRef CAS
.
- M. Sile-Yuksel, B. Tas, D. Y. Koseoglu-Imer and I. Koyuncu, Desalination, 2014, 347, 120–130 CrossRef CAS
.
- J. E. Kilduff, S. Mattaraj, J. P. Pieracci and G. Belfort, Desalination, 2000, 132, 133–142 CrossRef CAS
.
- I. Sadeghi, A. Aroujalian, A. Raisi, B. Dabir and M. Fathizadeh, J. Membr. Sci., 2013, 430, 24–36 CrossRef CAS
.
- V. Moghimifar, A. Raisi and A. Aroujalian, J. Membr. Sci., 2014, 461, 69–80 CrossRef CAS
.
- A. Sadeghnejad, A. Aroujalian, A. Raisi and S. Fazel, Surf. Coat. Technol., 2014, 245, 1–8 CrossRef CAS
.
- Y. Ren, C. Wang and Y. Qiu, Appl. Surf. Sci., 2007, 253, 9283–9289 CrossRef CAS
.
- X. Wei, B. Zhao, X.-M. Li, Z. Wang, B.-Q. He, T. He and B. Jiang, J. Membr. Sci., 2012, 407, 164–175 CrossRef
.
- B. Jaleh, P. Parvin, P. Wanichapichart, A. P. Saffar and A. Reyhani, Appl. Surf. Sci., 2010, 257, 1655–1659 CrossRef CAS
.
- H. Susanto, N. Stahra and M. Ulbricht, J. Membr. Sci., 2009, 342, 153–164 CrossRef CAS
.
- A. S. Dehnavi, A. Raisi and A. Aroujalian, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2013, 43, 543–551 CrossRef CAS
.
- J. F. Li, Z. L. Xu, H. Yang, C. P. Feng and J. H. Shi, J. Appl. Polym. Sci., 2008, 107, 4100–4108 CrossRef CAS
.
- G. T. Pyne, Biochem. J., 1932, 26, 1006–1014 CrossRef CAS PubMed
.
- A. Henglein, Chem. Mater., 1998, 10, 444–450 CrossRef CAS
.
- S. J. Shin, J. P. Kim, H. J. Kim, J. H. Jeon and B. R. Min, Desalination, 2005, 186, 1–10 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra23257d |
| This journal is © The Royal Society of Chemistry 2016 |



