Liquid metal soft electrode triggered discharge plasma in aqueous solution†
Abstract
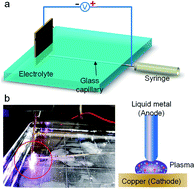
This paper reports a fundamental phenomenon whereby discharge plasma can be easily triggered in aqueous solution under a low voltage via a liquid metal electrode that is either static or a jetting stream. Plasmas with light emission are generated, which could last for several milliseconds each time, yet with a consistent current. The principal peaks of such optical emission spectrum lie in the blue, violet and ultraviolet sections, which are mainly caused by the plasma of gallium and indium. The influence of condition changes, such as voltage direction and solution constituents, on this phenomenon was also investigated. Further, this method to produce plasma has also been demonstrated to be useful for the fabrication of micro-sized metal particles or other compounds.

 Please wait while we load your content...
Please wait while we load your content...