Total protein concentration quantification using nanobeads with a new highly luminescent terbium(iii) complex†
Abstract
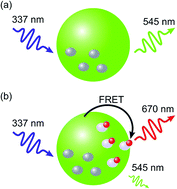
Total protein concentration (TPC) is a key parameter in many biochemical experiments and its quantification is often necessary for isolation, separation, and analysis of proteins. A sensitive and rapid nanobead-based TPC quantification assay based on Förster Resonance Energy Transfer (FRET) has been developed. A new, highly luminescent Tb(III) complex has been synthesised and applied as donor in this FRET assay with an organic dye (Cy5) as acceptor. FRET-induced changes in luminescence have been investigated both at donor and acceptor emission wavelength using time-resolved luminescence spectroscopy with time-gated detection. In the assay, the Tb(III) complex and fine-tuned polyglycidyl methacrylate (PGMA) nanobeads ensure that an improvement in sensitivity and background reduction is achieved. Using 40 nm large PGMA nanobeads loaded with the Tb(III) complex, it is possible to determine TPC down to 50 ng mL−1 in just 10 minutes. Through specific assay components the sensitivity has been improved when compared to existing nanobead-based assays and to currently known commercial methods. Additionally, the assay is relatively insensitive to the presence of contaminants, such as non-ionic detergents commonly found in biological samples. Due to no need for any centrifugal steps, this mix-and-measure bioassay can easily be implemented into routine TPC quantification protocols in biochemical laboratories.

 Please wait while we load your content...
Please wait while we load your content...