Lineariifolianoids I–L, four rare sesquiterpene lactone dimers inhibiting NO production from Inula lineariifolia†
Li-Ping Chen‡§
a,
Guo-Zhen Wu‡§a,
Hong-Yuan Donga,
Niao Yanga,
Wei-Dong Zhang*ab and
Hui-Liang Li*a
aDepartment of Phytochemistry, School of Pharmacy, Second Military Medical University, Shanghai 200433, P. R. China. E-mail: wdzhangy@hotmail.com; faranli@hotmail.com
bShanghai Institute of Pharmaceutical Industry, Shanghai 200040, P. R. China
First published on 25th October 2016
Abstract
A rare 4,5-seco-pseudoguaiane–guaiane type sesquiterpene lactone dimer lineariifolianoid I (1), together with three xanthane–guaiane type dimers lineariifolianoids J–L (2–4) were isolated from Inula lineariifolia. Their structures and absolute configurations were established on the basis of spectroscopic data and X-ray crystallography. Compounds 1 and 2 exhibited significant inhibitory activity against LPS-induced NO production in RAW 264.7 macrophages with IC50 values of 1.02, 1.79 μM.
The large genus Inula, consisting of about 100 species, is widely distributed throughout the world.1 The herb Inula lineariifolia has been used in traditional Chinese medicine as “JinFeiCao”.2 A large number of structurally diverse compounds, such as sesquiterpene lactone monomers and dimers, have been reported.2–6 In our previous works, eight sesquiterpene lactone dimers (SLDs), lineariifolianoids A–H, have been isolated from the aerial parts of I. lineariifolia.3,4 Among of them, lineariifolianoids A–D are four rare xanthane–guaiane type SLDs.
As a continuing work to enrich and deeply investigate pharmacological activities as well as the mechanism of those dimers, 500 kg the aerial parts of I. lineariifolia were collected and systematically isolated. Besides the known eight SLDs, lineariifolianoids I–L (1–4), further four new SLDs were identified. Compound 1 was an exo-Diels–Alder [4 + 2] adduct of a rare 4,5-seco-pseudoguaiane moiety and a guaiane moiety, while compounds 2–4, possessing the same carbon skeleton with lineariifolianoids A–C, were endo-Diels–Alder [4 + 2] adducts of xanthane moiety and guaiane moiety. Herein, we described the isolation, structure elucidation and NO inhibitory activities of 1–4.
According to our experience on the isolation of SLDs, the air-dried aerial parts of I. lineariifolia (500 kg) was extracted three times with 80% ethanol at room temperature to afford a crude extract (12.72 kg), which further partitioned by petroleum ether and CH2Cl2, giving a CH2Cl2-soluble fraction (6.86 kg). The CH2Cl2 fraction was segmented by silica gel (petroleum ether/EtOAC 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively) to give two fractions. The second fraction (P/E 1
1, respectively) to give two fractions. The second fraction (P/E 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.70 kg) was dissolved in 5 L EtOAC, and filtered to eliminate the large amount of crystal (Britanin, 1.12 kg, a major monomer in the title plant). The concentrated filtrate (510 g) was chromatographed over MCI gel (MeOH/H2O, from 40 to 90%) to give Fr.1–Fr.6. Subsequently, Fr.4–Fr.6 were purified by semi-preparative and preparative HPLC (CH3CN/H2O from 40 to 60%), yielding 1 (157 mg), 2 (12.75 g), 3 (86 mg) and 4 (114 mg) (Fig. 1).
1, 1.70 kg) was dissolved in 5 L EtOAC, and filtered to eliminate the large amount of crystal (Britanin, 1.12 kg, a major monomer in the title plant). The concentrated filtrate (510 g) was chromatographed over MCI gel (MeOH/H2O, from 40 to 90%) to give Fr.1–Fr.6. Subsequently, Fr.4–Fr.6 were purified by semi-preparative and preparative HPLC (CH3CN/H2O from 40 to 60%), yielding 1 (157 mg), 2 (12.75 g), 3 (86 mg) and 4 (114 mg) (Fig. 1).
Lineariifolianoid I (1) was obtained as a colourless orthorhombic crystal (in MeOH) with [α]25D −164.2 (c 0.048, CHCl3). Its molecular formula C32H42O8 was determined by positive HRESIMS at m/z 577.2788 ([M + Na]+, calcd 577.6603), indicating 12 degrees of unsaturation. Two characteristic absorptions at 1770 and 1727 cm−1 in the IR spectrum indicated the presence of ester carboxyl groups. The 13C NMR and DEPT spectra gave 32 carbon resonances including 5 methyls, 7 methylenes, 12 methines, and 8 quaternary carbons (Table S2, ESI†). From these resonances, one acetoxy could be clearly identified due to the signals at δC 171.4, 20.9 and δH 2.07 (3H, s). Deducting the carbon resonances for the acetoxy, the remain 30 carbon resonances containing one α-methylene-γ-lactone functionality (δH 6.18, d, J = 3.0 Hz; δH 5.46, d, J = 3.0 Hz; δC 169.6, 139.9 and 118.6) and one downfield shifted signal of carbonyl group of lactone (δC 184.1). These characteristic signals, with the MS data, suggested that 1 might be a dimeric sesquiterpene lactone.
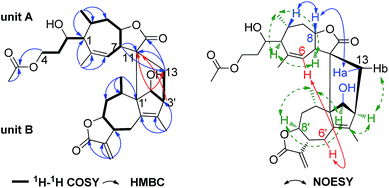
The planar structure of 1 was established by comprehensive analyses of the 1H–1H COSY and HMBC spectra as shown in Fig. 2. In unit A, two long proton-bearing chains of H-1/H-2/H2-3/H2-4 and H-6/H-7/H-8/H2-9/H-10/H3-14 were constructed by the 1H–1H COSY spectrum, combined with the HMBC correlations of H3-14/C-1, C-9, C-10 and H3-15/C-1, C-5, C-6 were established one seven membered ring with C3 lateral chain fragment. The HMBC correlation of H2-4 (δH 4.35, 4.13, 1H, respectively) with the C-1′′ at δC 171.4 confirmed that the acetoxy was attached to CH2-4. Moreover, the HMBC correlations of H-7/C-11, C-13 and H2-13/C-11, C-12 were constructed one lactone fragment. Thus, the two fragments of unit A was established a 4,5-seco-pseudoguaiane sesquiterpene lactone moiety which similar to deoxytetrahydroinulicin, a rare 4,5-seco-pseudoguaiane sesquiterpene isolated from Inula japonica Thunb. in 1971.7 Apart from the signals of unit A, the remaining ones construct the skeleton of unit B as a guaianolide moiety,8 based on the 1H–1H COSY correlations of H-2′/H-3′; H2-6′/H-7′/H-8′/H2-9′/H-10′/H3-14′ and the HMBC correlations of H2-13′/C-7′, C-11′, C-12′; H3-14′/C-9′, C-10′, C-1′ and H3-15′/C-3′, C-4′, C-5′. The linkage of the two units via two C–C single bonds between C-13/C-3′ and C-11/C-1′ was established by the 1H–1H COSY correlation of H2-13/H-3′/H-2′ and the key HMBC correlations of H-3′/C-11 and H-2′/C-11, C-13 (Fig. 2).9 Thus, the planar structure of 1 was constructed as shown in Fig. 2, probably formed from a Diels–Alder [4 + 2] cycloaddition reaction.
Obviously, the structure of 1 possesses 12 stereogenic centers. Except the C-2, the relative configuration of other 11 chiral carbons was concluded from NOESY correlations (Fig. 2). In unit A, the H-7 was arbitrarily assigned as the α-orientation, the NOESY correlations of H-1/H-7/H3-14, indicated that H-1, H-7, H3-14 were in the same face, while H-8, H-10 and H2-13 were on the other face and assigned as β-orientation. In unit B, the NOESY correlations of H-2′/H-3′/H3-14′/H-8′ implied that H-2′, H-3′, H-8′ and H3-14′ were assigned as α-orientation, while the H-10′ and the hydroxyl at C-2′ were assigned as β-orientation. Meanwhile, the significant NOESY correlations between H2-6′ and H-6 were found out, indicating that 1 was a staked arrangement of unit A and B (i.e., exo orientation). To further elucidate the configuration of C-2 and the absolute configuration of 1, it was necessary to execute a Cu Kα X-ray diffraction experiment. Fortunately, orthorhombic crystal was obtained from MeOH. The X-ray crystallographic analysis established unambiguously the absolute configuration of 1 was to be 1S, 2S, 7R, 8S, 10R, 11S, 1′S, 2′S, 3′S, 7′R, 8′S, 10′S, respectively. Consequently, the structure of 1 was fixed and named lineariifolianoid I (Fig. 3).
Lineariifolianoid J (2), a major SLD in I. lineariifolia, was also obtained as a colourless orthorhombic crystal (in MeOH) with [α]25D +186.3 (c 0.062, CHCl3), its molecular formula C34H42O10 was determined by positive HRESIMS at m/z 633.2678 ([M + Na]+, calcd 633.6805). Total 34 signals including one ketone carboxyl (δC 208.8) and four ester carboxyls (δC 180.1, 170.4, 170.2, 169.8) in the 13C NMR, combined with the presence of a typical α-methylene-γ-lactone, indicated that 2 should be a diacetoxyl xanthane–guaiane type SLD, closely related to the known compound lineariifolianoid C,3 which was previously isolated from the same plant in our laboratory. This deduction was proved by the key 1H–1H COSY and HMBC correlations as shown in Fig. 4. The positions of the two acetoxy were established by the HMBC correlations of H-6/C-1′′′ and H-2′/C-1′′, respectively.
The NOESY correlations indicated that most chiral carbons in 2 maintained the same configurations as those in lineariifolianoid C. For unambiguous confirmation the configuration of 2, X-ray diffraction data (Fig. 5) was performed, the absolute configuration of 2 was determined to be 2R, 6S, 7R, 8R, 10S, 11R, 1′S, 2′S, 3′R, 6′S, 7′S, 8′S, 10′R. Thus, the structure of lineariifolianoid J (2) was established as 2′-O-acetyl-lineariifolianoid C.
The molecular formula (C32H40O8) of compound 3 was assigned by HRESIMS. The IR spectrum and the 1H and 13C NMR data of 3 (Table S1 and S2, ESI†) implied that 3 was closely related to 2 except for only one acetoxyl group retains in 3. The residual acetoxyl group was located at C-2′ determined by the HMBC correlation of H-2′/C-1′′. Hence, the structure of 3 was assigned as 6-deacetoxyl derivative of 2 and named lineariifolianoid K.
Lineariifolianoid L (4) was obtained as optically active colourless oil with [α]25D +41.9 (c 0.062, CHCl3). The HRESIMS (m/z 575.2634, [M + Na]+) of compound 4 provided the same molecular formula C32H40O8 as 3. The 1H and 13C NMR data of 4 resembled those of 3, except a hydroxy group substituted at C-6 in 4 instead of at C-6′ in 3, which were supported by the 1H–1H COSY correlations of H-5/H-6/H-7/H-8/H-9/H-10/H3-14. Meanwhile, the NOESY correlation of H-6/H-7 suggested the hydroxyl at C-6 was β-orientation. Thus, the structure of 4 was determined and named lineariifolianoid L.
In traditional Chinese medicine, I. lineariifolia was used to cure inflammatory as “JinFeiCao”. Most of sesquiterpene lactone monomers and dimers from Inula genus exert significant anti-inflammatory activity.2,10 Therefore, the inhibitory activities against LPS-induced nitric oxide (NO) production in RAW 264.7 macrophages of 1–4 were evaluated.11 Compounds 1 and 2 exhibited significant inhibitory effects with IC50 values of 1.02, 1.79 μM, as well as 3 and 4 showed moderate inhibitory effects with IC50 values of 10.02, 10.16 μM, respectively (Table S3, ESI†). Furthermore, compounds 1–4 were not significantly cytotoxic at the concentrations required for inhibition NO production (as defined by MTT assay).12
In conclusion, lineariifolianoid I (1), a rare unsymmetrical SLD comprising of 4,5-seco-pseudoguaiane and guaiane units with the linkage mode of two C–C single bonds between C-13/C-3′ and C-11/C-1′, has been isolated and elucidated for the first time. Lineariifolianoid J (2, 12.75 g), a major SLD from I. lineariifolia, exhibited significant inhibitory activity against LPS-induced NO production in RAW 264.7 macrophages with IC50 values of 1.79 μM. These results will provide scientific foundation for rational development and utilization of this plant, and might supply information for the future design of anti-inflammatory agents.
Acknowledgements
This work was supported by the Professor of Chang Jiang Scholars Program, National Nature Science Foundation of China (81102335, 81230090, 81473109, 81502957), National High-Tech Research and Development Program of China (863 Program, 2014AA022201-03), Scientific Foundation of Shanghai China (13401900103), Shanghai Engineering Research Center for the Preparation of Bioactive Natural Products (16DZ2280200) and the China Postdoctoral Science Foundation funded project (2015M572740).Notes and references
- S. Amin, Z. A. Kaloo, S. Singh and T. Altaf, Int. J. Adv. Res., 2013, 1, 20–26 Search PubMed.
- L.-Y. Nie, J.-J. Qin, H. Ying, Y. Lan, Y.-B. Liu and Y.-X. Pan, J. Nat. Prod., 2010, 73, 1117–1120 CrossRef CAS PubMed.
- J.-J. Qin, Y. Huang, D. Wang, X.-R. Cheng, Q. Zeng and S.-D. Zhang, RSC Adv., 2012, 2, 1307–1309 RSC.
- J.-J. Qin, H.-Z. Jin, Y. Huang, S.-D. Zhang, L. Shan and S. Voruganti, Eur. J. Med. Chem., 2013, 68, 473–481 CrossRef CAS PubMed.
- G.-W. Wang, J.-J. Qin, X.-R. Cheng, Y.-H. Shen, L. Shan and H.-Z. Jin, Expert Opin. Invest. Drugs, 2014, 23, 317–345 CrossRef CAS PubMed.
- L.-Y. Nie, H.-Z. Jin, L. Yan, J.-J. Qin and W.-D. Zhang, Nat. Prod. Res. Dev., 2011, 23, 643–646 CAS.
- E. Y. Kiseleva, V. I. Sheichenko, K. S. Rybalko, G. A. Kalabin and A. I. Ban'Kovskii, Chem. Nat. Compd., 1971, 7, 254–259 CrossRef.
- C. Zdero, F. Bohlmann, R. M. King and H. Robinson, Phytochemistry, 1988, 27, 2835–2842 CrossRef CAS.
- X.-K. Xu, J. Ye, L.-P. Chen, W.-D. Zhang, Y.-X. Yang and H.-L. Li, Tetrahedron Lett., 2015, 56, 6381–6384 CrossRef CAS.
- J.-J. Qin, J.-X. Zhu, Q. Zeng, X.-R. Cheng, Y. Zhu and S.-D. Zhang, J. Nat. Prod., 2011, 74, 1881–1887 CrossRef CAS PubMed.
- S. Kanno, M. Kakuta, Y. Kitajima, Y. Osanai, K. Kurauchi and T. Ohtake, J. Pharmacol. Sci., 2007, 104, 278–281 CrossRef CAS.
- S. I. Kanno, S. Ai, R. Hirata, K. Asou and M. Ishikawa, J. Pharmacol. Sci., 2004, 75, 353–365 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: The extraction scheme, compounds characterization, spectroscopic data, bioassay methods, and crystallographic data of 1 and 2. CCDC 1495848 and 1493163. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra23092j |
| ‡ These authors contributed equally to this work. |
| § The authors declare no competing financial interest. |
| This journal is © The Royal Society of Chemistry 2016 |