Facile hydrothermal synthesis and multicolor-tunable luminescence of YPO4:Ln3+ (Ln = Eu, Tb)†
Hailong Xiong,
Jianchao Dong,
Junfeng Yang,
Yali Liu,
Hongbo Song and
Shucai Gan*
College of Chemistry, Jilin University, Changchun 130026, PR China. E-mail: gansc@jlu.edu.cn
First published on 10th October 2016
Abstract
Well-crystallized tetragonal YPO4 and YPO4:Ln3+ (Ln = Eu, Tb) nanocrystals with cuboid morphology and narrow size distribution have been successfully synthesized through a facile two-step hydrothermal synthesis route using Y4O(OH)9NO3 as a sacrificial precursor for the first time. The structure, morphology and phase evolution process were significantly affected by the reaction conditions (hydrothermal treatment time and concentration of (NH4)2HPO4). The luminescence colors of YPO4:Tb3+, Eu3+ phosphors can be easily tuned from green to primrose yellow and then to white by adjusting the concentration of Eu3+ ions under 373 nm irradiation. Furthermore, the energy transfer efficiency of YPO4:0.08Tb3+, xEu3+ increased gradually and reached about 94.30% when the concentration of Eu3+ ions was 0.10. The energy transfer mechanism from Tb3+ to Eu3+ has been proved to be a quadrupole–quadrupole interaction mechanism. These merits manifest that the yttrium phosphate phosphors with tunable white light emission may have potential applications in white light-emitting diodes.
1 Introduction
In recent years, hydrothermal synthesis has drawn comprehensive attention, due to its superior characteristics, such as controllable reaction conditions, simple facilities, relatively high yield and low reaction cost, as well as frequent use of water as the reaction medium.1 As one of the most effective solution-based approaches, hydrothermal synthesis has been of great importance for preparing novel lanthanide (Ln3+)-doped nano/micro luminescent materials.2–4 Especially hydrothermal conversion method has been widely employed. For example, Jia et al.5 prepared octahedral LuVO4 microcrystals through a designed two-step hydrothermal method using Lu4O(OH)9NO3 as a sacrificial precursor. Furthermore, it has been universally acknowledged that the properties of inorganic nano/micro luminescent materials, to some extent, are not only related to their chemical composition and crystal structure, but also their size, shape, and dimensionality.6,7 Up to now, considerable efforts for hydrothermal synthesis have been dedicated to designing various crystal structures, such as churchite, monazite, rhabdophane and zircon,8–10 and morphologies (e.g., nanotubes,11 nanorods,12 nanofibers,13 nanosheets14). In particular, organic additives have been extensively used to control the dynamics of crystal growth and manipulate the shape of materials. However, the additives may result in unavoidable surface contamination and incur impurities in the obtained samples, and the kinetics of the crystal growth with additives is a complicated process. Therefore, it still remains a challenge to develop a mild and environmentally friendly reaction system for fabrication of size and shape controlled nano/microluminescent materials without any organic solvent, surfactant, or catalyst.Lanthanide orthophosphates (REPO4) have been widely investigated,15–17 due to their high thermal and chemical stability,18 refractive index19 and quantum yield.20 They have been extensively used in various fields,21,22 such as high quality phosphors, high-performance luminescent devices, magnets, optoelectronics, catalysts, solar cells, down and up-conversion materials, and other functional materials, on account of their fascinating optical, catalytic, and magnetic characteristics. Among the different lanthanide phosphates researches, yttrium orthophosphate with unprecedented luminescent functions is chosen as a host matrix and Eu3+, Tb3+ as dopants to study the luminescence properties, based on the following characteristics:23,24 (1) It is isostructural to metal phosphates (LnPO4). Y3+ sites can be easily substituted by Ln3+ ions because of similar ionic size and oxidation state, (2) the host has high thermal stability, chemical stability and good biocompatibility. More importantly, (3) YPO4 has several phases like hexagonal, monoclinic and tetragonal. These merits indicate that YPO4 is a better host. Therein, the hexagonal phase is metastable and can be apt to transform to the thermodynamically stable phases (monoclinic phase and tetragonal phase) by high-temperature calcination, but the reverse process is very difficult.25 For example, Luwang et al.26 reported that the tetragonal phase YPO4 transformed to hexagonal structure by increasing Ce3+ concentration, and then returned to the tetragonal structure on annealing above 900 °C. However, there are still few reports on phase transformation from thermodynamically stable phases to metastable phases and reversely to thermodynamically stable phases without any organic additives in the hydrothermal system. If phase evolution could be suitably adjusted by hydrothermal conditions, many attractive properties would be realizable. Therefore, we are working to solve those aforementioned problems.
In this work, via a facile hydrothermal conversion method, a series of Eu3+, Tb3+ and Tb3+/Eu3+ co-doped YPO4 phosphors have been successfully synthesized by treating Y4O(OH)9NO3 precursor with diammonium hydrogen phosphate for the first time. The effects of reaction conditions on structure, morphology and phase transformation process of as-synthesized samples have been studied in detail. Moreover, the photoluminescence (PL) properties of Eu3+, Tb3+ and Tb3+/Eu3+ co-doped YPO4 phosphors have also been investigated.
2 Experimental section
2.1 Materials
Y(NO3)3 and Ln(NO3)3 (Ln = Eu and Tb) aqueous solutions were prepared by dissolving the corresponding metal oxide (99.99%) in dilute nitric acid solution under heating with agitation, respectively. Diammonium hydrogen phosphate (analytic reagent) was purchased from Tianjin Kermel Chemical Reagent Co., Ltd. Other chemicals were purchased from Beijing Beihua Fine Chemicals Co. All the other chemical reagents were of analytical grade and used directly without further purification.2.2 Hydrothermal synthesis of Y4O(OH)9NO3 precursor
The Y4O(OH)9NO3 precursor were prepared via a straightfoward hydrothermal synthetic process. 20 mL Y(NO3)3 (0.1 M) aqueous solution was added to 15 mL distilled water. Then 25 wt% aqueous ammonia solution was added dropwise into the solution with continuous agitation until pH = 9.5. After homogenizing for 30 min, a white colloidal precipitate (total volume = 35 mL) was formed, which was transferred into a 50 mL stainless Teflon-lined autoclave and heated at 180 °C for 24 h. After the autoclave was naturally cooled to room temperature, the white precipitate were collected by centrifugation, washed with distilled water and absolute ethanol several times to remove by-products, and finally dried in air at 60 °C for 12 h.2.3 Preparation of the cuboid YPO4 samples
The 1 mmol as-prepared precursor suspension was firstly redispersed into 10 mL distilled water by ultrasonic treatment. Simultaneously, a certain quantity (NH4)2HPO4 was dissolved in 30 mL distilled water to form a colorless solution. And then mixed the above two solutions, followed by additional agitation for 20 min, the resultant mixture was transferred to a 50 mL autoclave, sealed and maintained at 180 °C for 72 h. Subsequently, the resulting product was cooled down to room temperature naturally. The precipitate was separated by centrifugation, sequentially washed with deionized water and anhydrous ethanol several times, and dried in air at 70 °C for 12 h. Different reaction time (24, 36, 48, 72 h) and reactant concentrations of diammonium hydrogen phosphate (2, 4, 6, 8 mmol) were selected to investigate the conversion process from precursor to final product. A similar process was repeated under similar manner for the synthesis of YPO4:Ln3+ (Ln = Eu, Tb) samples, by using the same amount of Ln(NO3)3 solutions instead of Y(NO3)3 as the starting materials.2.4 Characterization
Phase identification was made via X-ray powder diffraction (XRD) performed on a Rigaku D/max-II B X-ray diffractometer with monochromatic Cu Kα radiation (wavelength λ = 1.5418 Å). The XRD data were collected using a scanning mode in the 2θ range from 10° to 70° with a scanning step size of 0.02° and a scanning rate of 4.0° per minute. The morphology and microstructure of the as-obtained products were analyzed by field emission scanning electron microscopy (FE-SEM, S4800, Hitachi) operated under 15 kV. The ultraviolet-visible PL excitation and emission spectra were analyzed with a Hitachi F-7000 spectrophotometer equipped with a 150 W xenon lamp as an excitation source. All the measurements were performed at room temperature.3 Results and discussion
3.1 Phase structure, and morphology
The crystal structure and phase purity of the as-obtained samples were detected by XRD measurements. Different reaction time and reagent concentrations of diammonium hydrogen phosphate were investigated for the synthesis of single phase YPO4 products in present system. Fig. 1a shows the X-ray diffraction pattern of the as-obtained yttrium precursor. It is self-evident that well-defined diffraction peaks can be readily indexed to the monoclinic phase of Y4O(OH)9NO3 (JCPDS no. 79-1352). In order to investigate the possible formation process of hydrothermal conversion from precursor compound to target product, time-dependent XRD experiments were performed (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of (NH4)2HPO4/precursor) and other preparation conditions kept constant. When the reaction time was carried out for 24 h, the tetragonal phase appeared and became the dominant phase in comparison with the precursor (Fig. 1b), the diffraction peaks of the product can be indexed as a mixture of the precursor and tetragonal phase (JCPDS no. 83-0658). As the reaction time increased to 36 h (Fig. 1c), it can be seen that the diffraction peaks of the Y4O(OH)9NO3 precursor have almost disappeared except for the main peak at 10.08°. In addition, some strong diffraction peaks belonging to the hexagonal phase of YPO4·0.8H2O (JCPDS no. 42-0082) could be detected and became the prime phase. It can be indicated that most of the precursor have transformed to more stable phase during the hydrothermal process. With the increase of the reaction time to 48 h, the diffraction peaks matched well the tetragonal phase besides some minor peaks of hexagonal phase at 14.9°, 20.6°, 30.2°, 32.2°, 43.2°, respectively. Upon further increasing the time to 72 h (Fig. 1d), both the residual precursor and YPO4·0.8H2O disappeared completely, all diffraction peaks matched well a tetragonal phase, while no other peaks from impurities can be observed, which indicates a pure tetragonal phase. Moreover, the diffraction peaks are very intense and sharp, indicating that the powder samples with high crystallinity can be prepared by hydrothermal conversion method. It is extremely essential for phosphors, because high crystallinity generally means fewer defects and stronger luminescence.
1 molar ratio of (NH4)2HPO4/precursor) and other preparation conditions kept constant. When the reaction time was carried out for 24 h, the tetragonal phase appeared and became the dominant phase in comparison with the precursor (Fig. 1b), the diffraction peaks of the product can be indexed as a mixture of the precursor and tetragonal phase (JCPDS no. 83-0658). As the reaction time increased to 36 h (Fig. 1c), it can be seen that the diffraction peaks of the Y4O(OH)9NO3 precursor have almost disappeared except for the main peak at 10.08°. In addition, some strong diffraction peaks belonging to the hexagonal phase of YPO4·0.8H2O (JCPDS no. 42-0082) could be detected and became the prime phase. It can be indicated that most of the precursor have transformed to more stable phase during the hydrothermal process. With the increase of the reaction time to 48 h, the diffraction peaks matched well the tetragonal phase besides some minor peaks of hexagonal phase at 14.9°, 20.6°, 30.2°, 32.2°, 43.2°, respectively. Upon further increasing the time to 72 h (Fig. 1d), both the residual precursor and YPO4·0.8H2O disappeared completely, all diffraction peaks matched well a tetragonal phase, while no other peaks from impurities can be observed, which indicates a pure tetragonal phase. Moreover, the diffraction peaks are very intense and sharp, indicating that the powder samples with high crystallinity can be prepared by hydrothermal conversion method. It is extremely essential for phosphors, because high crystallinity generally means fewer defects and stronger luminescence.
It is worth noting that the phase transformation under hydrothermal conditions are considerably different from those upon high-temperature reactions. In this work, the unordinary mixed phase evolution from tetragonal phase to hexagonal phase occurred and then transformed to pure tetragonal phase during hydrothermal process. It is difficult to understand the phase transformation via thermodynamic process, because tetragonal phase is more stable than hexagonal phase in water system.24 The metastable phases are apt to transform to the thermodynamically stable phases by high-temperature calcination. Luwang et al.27 reported that the phase evolution from hexagonal to tetragonal phase when the annealing temperature reached 800 °C in the Bi3+ and Eu3+ co-doped YPO4 samples. Zollfrank et al.28 reported that the phase transformation from monoclinic nonhydrated EuPO4 to monazite when temperature exceeding 600 °C. However, the reverse process is very difficult, and even impossible without the use of any surfactant, additive or catalyst. Zhao et al.25 found that low-temperature monoclinic BiPO4 reversely transformed to hexagonal phase when the hydrothermal temperature increased above 180 °C. For the latter case, they claimed that the transformation process might be ascribed to the reaction medium of water. However, Zhao et al. didn't study the effect of reaction time and high PO43− ions concentration on phase transformation process. In order to understand the possible mechanism of phase evolution, a series of parallel experiments with different molar ratio (P/Y) of (NH4)2HPO4/precursor (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6
1, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8
1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were studied deeply. Fig. S1† compares XRD patterns of the samples synthesized with different molar ratios, as shown in Fig. S1a and b,† the product prepared at P/Y = 2 and 4, remained numerous precursors, which indicated the lack of PO43− ions. With raising the P/Y value to 6 (Fig. S1c†), the hexagonal phase appeared, interestingly. Upon further extending the P/Y value to 8 (Fig. S1d†), all diffraction peaks matched well a pure tetragonal phase. This result adequately demonstrated that different reactant concentrations had a substantial impact on the evolution of crystal structure.
1) were studied deeply. Fig. S1† compares XRD patterns of the samples synthesized with different molar ratios, as shown in Fig. S1a and b,† the product prepared at P/Y = 2 and 4, remained numerous precursors, which indicated the lack of PO43− ions. With raising the P/Y value to 6 (Fig. S1c†), the hexagonal phase appeared, interestingly. Upon further extending the P/Y value to 8 (Fig. S1d†), all diffraction peaks matched well a pure tetragonal phase. This result adequately demonstrated that different reactant concentrations had a substantial impact on the evolution of crystal structure.
In order to elaborate the formation mechanism of the cuboid YPO4 samples in detail, time-dependent experiments were carried out by keeping other reaction parameters unchanged, which is showed in Fig. 2. Fig. S2† is a panoramic SEM image observed from the as-formed Y4O(OH)9NO3, clearly indicating that the precursor is totally composed of uniform nanorods with diameters of 200 nm and lengths of about 1 μm. When the precursor reacted with (NH4)2HPO4 at 24 h (Fig. 2a), it can be seen that the as-obtained samples inherited its parents' morphologies, and a large number of cuboid YPO4 samples formed on the surface of precursor, which resulted in roughness and the bigger size of precursor due to the decomposition of the precursor and crystallization of the YPO4. When the reaction time extended to 36 h (Fig. 2b), it was obvious that abundant precursor disintegrated into cuboid nanostructures and some irregular nanocrystals. By increasing the reaction time from 36 h to 48 h (Fig. 2c), the precursor disappeared and completely decomposed to cuboid YPO4 besides a few irregular nanocrystals. The homogeneous and uniform cuboid YPO4 products (the diameters of about 100 nm and lengths of about 240 nm) could be acquired when the reaction time prolonged to 72 h (Fig. 2d). According to the above experimental results and analysis, a schematic illustration for the formation of the YPO4 sample is presented in Fig. S3.† Moreover, it could be observed that the surfaces of the cuboid YPO4 were very smooth, implying less flaws and more intense luminescence, which was in agreement with the XRD results of the sample.
 | ||
| Fig. 2 SEM images of the hydrothermal conversion products prepared at 180 °C for 24 h (a), 36 h (b), 48 h (c), and 72 h (d). | ||
On the basis of the results and analysis mentioned above, it is possible to interpret the conversion process as follows. In the early stage of the reaction, the precursor disintegrated slowly and limited Y3+ ions generated in the system, resulting in a slow reaction rate, which is conducive to form tetragonal phase. With the increase of reaction time, the dissolution rate of the precursor also increased, and more free Y3+ ions were released in the solution, which increased the reaction rate under the high PO43− concentration. During the reaction process, the Y3+ ions near the nuclei were consumed quickly, which led to a considerably low concentration of Y3+ ions in the local micro regions. As a result, a high concentration difference formed between the micro regions near the nanocrystals and bulk solution. Consequently, the mass diffusion of Y3+ ions is the key to the succedent formation of crystals.29 This result allows us to conjecture that the excess PO43− ions concentration results in higher reaction rate, which may be favorable for the formation of hexagonal phase instead of tetragonal phase. Vanetsev et al.30 also reported that the high PO43− ions concentration would fasten chemical reaction rate and be in favor of forming hexagonal phosphate. As the reaction time further increased, the Y4O(OH)9NO3 precursor was completely consumed, and the hexagonal phase entirely converted into pure tetragonal phase. This changing process is suitable for thermodynamic process. Based on these analyses, the above transformation might be associated with reaction time and excess phosphate ions.
3.2 Luminescence properties
It is generally acknowledged that lanthanide orthophosphate is very remarkable host matrix, which is used as luminescent material with manifold optically active lanthanide ions. Among the lanthanide ions, Eu3+ ion is a crucial red-emitting activator in commercial phosphors, which is ascribed to the 5D0 → 7FJ (J = 1–6) transitions in the red spectral area. Moreover, Tb3+ ion emits blue light stemming from the transitions of 5D3 → 7FJ (J = 1 and 2) and green light resulting from the transitions of 5D4 → 7FJ (J = 3–6), which is used as an activator in green phosphors.5 In order to achieve the multicolour tunable luminescence, Eu3+, Tb3+and Tb3+/Eu3+ co-doped YPO4 phosphors have been synthesized in our experiment.Fig. 3a shows the photoluminescence excitation (PLE) and photoluminescence emission (PL) spectra of YPO4:0.04Eu3+ phosphor. As seen from in Fig. 3a, the PLE spectra monitored at 592 nm presents several strong bands at 365 (7F0 → 5D4) nm, 385 (7F0 → 5L7) nm, 397 (7F0 → 5L6) nm, 419 (7F0 → 5D3) nm and 467 (7F0 → 5D2) nm in the 350–500 nm range, respectively. The incisive excitation peak at 397 nm is attributed to the intraconfigurational f–f transitions of Eu3+ ions, which is well associated with the comprehensively applied output wavelengths of UV LED chips. Upon UV excitation at 397 nm, the emission spectrum of YPO4:0.04Eu3+ makes up a few characteristic transitions of Eu3+ within its 4f6 configuration, corresponded to 5D0 → 7F1 (592 nm), 5D0 → 7F2 (618 nm), 5D0 → 7F3 (650 nm), 5D0 → 7F4 (695 nm), respectively. As everyone knows, the 5D0 → 7F2 hypersensitive transition (ΔJ = 2) belongs to the forced electric dipole transition, and it is easily affected by the chemical environment surrounding of Eu3+. While the 5D0 → 7F1 corresponds to the magnetic dipole transition, which is difficultly influenced by the crystal field around Eu3+ ions.31 In the emission spectrum of as-prepared YPO4:Eu3+ nanocrystals, the emission intensity at 592 nm is stronger than that at 617 nm. The result suggests that the Eu3+ ions in YPO4 occupied sites with inversion symmetry. What's more, each emission is observed as two subpeaks by reason of Stark energy splitting, which is influenced by the crystal field around Eu3+ environment in the host lattice.32 The quantum yield of YPO4:0.04Eu3+ phosphor is 33.02%.
Fig. 3b displays the PLE and PL spectra of YPO4:0.08Tb3+ phosphor. The excitation spectrum observed at 544 nm exhibits several strong peaks at 355 nm, 362 nm, 373 nm, 381 nm, 491 nm, corresponded to 7F6 → 5D2, 7F6 → 5L10, 7F6 → 5G5, 7F6 → 5G6 and 7F6 → 5D4, respectively. Upon excitation with 373 nm irradiation, the YPO4:0.08Tb3+ sample shows four prominent lines centered at 489 nm, 544 nm, 588 nm, 621 nm, which are associated with the transitions from the 5D4 excited state to the 7FJ (J = 6, 5, 4, 3) ground states of Tb3+ ions. The most highlighted peaks at 544 nm is ascribed to the 5D4 → 7F5 transition. Furthermore, the quantum yield of YPO4:0.08Tb3+ phosphor is tested to be 14.3%.
The doping concentrations of luminescent centers is a crucial factor affecting the phosphor performance.33 As a consequence, a series of Tb3+ doped YPO4 phosphors have been synthesized with different Tb3+ concentration to confirm the optimal dopant amount. As shown in Fig. 4, the emission spectra measured at 373 nm doesn't observe noteworthy shift of the peak positions with adjusting the Tb3+ ions concentration. However, the intensities of band have changed obviously. All the samples show the intense emission peaks ranging from 400 to 700 nm, the emission intensities increase with increasing the Tb3+ ions concentrations and reach a maximum at y = 0.08, and then decrease with further increasing (y) (the inset in Fig. 4) owning to concentration quenching.
Energy transfer is of great importance to improve the emission efficiency for nano/micro luminescent materials. In order to obtain the multicolour tunable luminescence, we choose the Tb3+ as sensitizer and Eu3+ as activator to investigate the energy transfer from Tb3+ to Eu3+. Fig. 5a compares the PLE spectrum of YPO4:Eu3+ and PL spectrum of YPO4:Tb3+. It can be seen clearly that a effective spectral overlap between the emission spectrum of Tb3+ and the excitation spectrum of Eu3+ is observed, which allows us to speculate that an efficient resonance type energy transfer may exist in the Tb3+/Eu3+ co-doped YPO4 phosphors. In order to further demonstrate the existence of the energy transfer from Tb3+ to Eu3+, when exciting with 355 nm laser light corresponding to Tb3+:7F6 → 5D2 transition (Fig. 5b), the PL spectrum concurrently contains the weak peaks 5D0 → 7F1 (592 nm), 5D0 → 7F2 (618 nm) of Eu3+ ions and the strong peaks 5D4 → 7F6 (489 nm), 5D4 → 7F5 (544 nm) transition of Tb3+ ions in the YPO4:0.08Tb3+, 0.04Eu3+ phosphor. Nevertheless, YPO4:0.04Eu3+ phosphor has no emissions because there is no obvious absorption of Eu3+ at 355 nm (Fig. 3a). In addition, upon 381 nm excitation (5G6 of Tb3+), compared with the emission spectrum of Eu3+ single-doped sample (Fig. 5c), some new emission peaks of Tb3+ can be observed in Tb3+/Eu3+ codoped sample. Moreover, in the YPO4:0.08Tb3+, 0.04Eu3+ sample, the emission intensities of Eu3+:5D0 → 7F1, 5D0 → 7F2 transitions obviously increase. While the emission intensity of 5D4 → 7F5 (544 nm) transition of Tb3+ ions distinctly decreases compared with the YPO4:0.08Tb3+ nanocrystal. The abovementioned spectral features give direct evidence to illustrate that the energy transfer process from Tb3+ to Eu3+ exists in YPO4 host.34,35
Fig. 6 illustrates the variation of the emission spectra of the YPO4:0.08Tb3+, xEu3+ (x = 0–0.10) phosphors. Under directly excited at 373 nm (7F6 → 5G5 excitation transition of Tb3+), the emission spectra shows the characteristic emission bands of both Tb3+ and Eu3+. With the increase of the Eu3+ content, the emission intensity of the sensitizer Tb3+ is found to decrease monotonically, due to the markedly energy transfer between Tb3+ and Eu3+. Meanwhile, the emission intensity of the activator Eu3+ first increases up to an optimum concentration at 0.04 and then decreases because of the concentration quenching effect. Moreover, the quantum yield of YPO4:0.08Tb3+, 0.04Eu3+ phosphor is 11.01%.
The energy transfer efficiency (ηT) from the sensitizer Tb3+ to the activator Eu3+ was also investigated as a function of Eu3+ content, which is depicted in Fig. 7. The energy transfer efficiency can be approximately estimated according to the following equation:36
 | (1) |
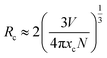 | (2) |
 | ||
| Fig. 7 The dependence of energy transfer efficiency ηT of YPO4:0.08Tb3+, xEu3+ phosphors on Eu3+ ions concentration. | ||
To further explain the nature of energy transfer mechanism from the Tb3+ to Eu3+ ions, Reisfeld's approximation and Dexter's energy transfer expressions of multipolar interaction are employed. The corresponding formula can be expressed by:38
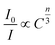 | (3) |
Fig. 9 shows the schematic illustration of energy transfer from Tb3+ to Eu3+. Upon 373 nm excitation, it can be seen that the energy level of Eu3+ (5D0,1) is located below energy level of Tb3+ (5D4). In addition, there is a effective overlap between 5D4 → 7FJ (J = 1–6) emission of Tb3+ and the 7F1 → 5D1,2 absorption of Eu3+, which indicates that energy transfer is valid. Therefore, the ground state electrons of Eu3+ ions may be excited to the excited state 5D1 by energy transfer from Tb3+ ions. Subsequently, the 5D1 energy level of Eu3+ relaxes to the 5D0 energy level, and finally transfers to the 7FJ (J = 1–4) ground states, which improves the characteristic emission of Eu3+.
 | ||
| Fig. 9 Schematic energy level diagram standing for the energy transfer and energy transfer mechanism in the Tb3+/Eu3+ co-doped YPO4 phosphors. | ||
Fig. 10 shows the calculated CIE chromaticity coordinates of YPO4:0.08Tb3+, xEu3+ samples under excitation at 373 nm, and the corresponding data in detail are listed in Table 1. The color tone of phosphors can be modulated from green to primrose yellow and then to white by adjusting the doped concentration of Eu3+ ions from 0 to 0.08. Furthermore, the chromaticity coordinate of YPO4:0.08Tb3+, 0.06Eu3+ phosphor is (0.335, 0.346), which is close to that of the standard white light (0.33, 0.33). The correlated color temperature (CCT) of the samples has also been calculated and the corresponding data are given in Table 1. Based on the above results, the yttrium phosphate phosphors with tunable white light emission may have potential application as white-emitting phosphors in white-LEDs.
 | ||
| Fig. 10 The CIE chromaticity diagram of the YPO4:0.08Tb3+, xEu3+ (x = 0, 0.02, 0.04, 0.06, 0.08) phosphors. | ||
| Label | Sample compositions | CIE (x, y) | CCT (K) |
|---|---|---|---|
| a | YPO4:0.08Tb3+ | (0.260, 0.505) | 7250 |
| b | YPO4:0.08Tb3+, 0.02Eu3+ | (0.292, 0.439) | 6690 |
| c | YPO4:0.08Tb3+, 0.04Eu3+ | (0.325, 0.405) | 5737 |
| d | YPO4:0.08Tb3+, 0.06Eu3+ | (0.335, 0.346) | 5387 |
| e | YPO4:0.08Tb3+, 0.08Eu3+ | (0.357, 0.338) | 4483 |
4 Conclusion
In summary, for the first time, we developed a facile hydrothermal conversion method for the synthesis of uniform and monodispersed cuboid YPO4 samples using Y4O(OH)9NO3 as a sacrificial precursor. The reaction conditions (hydrothermal treatment time and concentration of (NH4)2HPO4) play crucial roles in obtaining pure YPO4 products with uniform cuboid morphology and high crystallinity. Interestingly, it can be observed that the abnormal mixed phase evolution from tetragonal to hexagonal occurred, and then transformed to pure tetragonal phase by changing the reaction conditions. The energy transfer process from Tb3+ to Eu3+ has also been systematically investigated. The energy transfer mechanism between Tb3+ and Eu3+ has been demonstrated to be a quadrupole–quadrupole interaction. Moreover, the energy transfer efficiency (ηT) and average donor–acceptor distance (Rc) were calculated to be 94.30% and 10.46 Å, respectively. The emitting colors of Tb3+/Eu3+ co-doped YPO4 phosphors could be adjusted from green to the primrose yellow and then to white by tuning the Eu3+ doped concentration. These results reveal that this kind of material with variable and tunable colors could be potentially used in white-LEDs.Acknowledgements
This present work was financially supported by China Geological Survey (Grant No. 12120113088300); and the Key Technology and Equipment of Efficient Utilization of Oil Shale Resources, No. OSR-5.Notes and references
- R. Q. Li, Y. L. Liu, N. N. Zhang, L. L. Li, L. Liu, Y. M. Liang and S. C. Gan, J. Mater. Chem. C, 2015, 3, 3928–3934 RSC.
- Y. Wang, S. L. Gai, N. Niu, F. He and P. P. Yang, Phys. Chem. Chem. Phys., 2013, 15, 16795–16805 RSC.
- S. L. Gai, C. X. Li, P. P. Yang and J. Lin, Chem. Rev., 2014, 114, 2343–2389 CrossRef CAS PubMed.
- G. Jia, H. P. You, Y. H. Song, J. J. Jia, Y. H. Zheng, L. K. Zhang, K. Liu and H. J. Zhang, Inorg. Chem., 2009, 48, 10193–10201 CrossRef CAS PubMed.
- G. Jia, H. P. You, L. H. Zhang, Y. H. Zheng, K. Liu, Y. J. Huang and H. J. Zhang, CrystEngComm, 2009, 11, 2745–2750 RSC.
- H. Zheng, B. J. Chen, H. Q. Yu, J. S. Sun, X. P. Li, J. S. Zhang, H. Zhong, Z. L. Wu and H. P. Xia, RSC Adv., 2015, 5, 56337–56347 RSC.
- L. Zhang, L. L. Fu, X. X. Yang, Z. L. Fu, X. D. Qi and Z. J. Wu, J. Mater. Chem. C, 2014, 2, 9149–9158 RSC.
- H. Lai, Y. Du, M. Zhao, K. N. Sun and L. Yang, RSC Adv., 2014, 4, 50731–50738 RSC.
- C. X. Li, Z. Y. Hou, C. M. Zhang, P. P. Yang, G. G. Li, Z. H. Xu, Y. Fan and J. Lin, Chem. Mater., 2009, 21, 4598–4607 CrossRef CAS.
- C. S. Pan, D. Li, X. G. Ma, Y. Chen and Y. F. Zhu, Catal. Sci. Technol., 2011, 1, 1399–1405 CAS.
- L. Liu, N. N. Zhang, Z. H. Leng, Y. M. Liang, R. Q. Li, L. C. Zou and S. C. Gan, Dalton Trans., 2015, 44, 6645–6654 RSC.
- G. C. Park, S. M. Hwang, J. H. Lim and J. Joo, Nanoscale, 2014, 6, 1840–1847 RSC.
- X. Li, Y. Y. Li, X. Y. Chen, B. B. Li, B. Gao, Z. H. Ren, G. R. Han and C. B. Mao, Langmuir, 2016, 32, 3226–3233 CrossRef CAS PubMed.
- Z. H. Wang, J.-G. Li, Q. Zhu, X. D. Li and X. D. Sun, RSC Adv., 2016, 6, 22690–22699 RSC.
- M. Saraf, P. Kumar, G. Kedawat, J. Dwivedi, S. A. Vithayathil, N. Jaiswal, B. A. Kaipparettu and B. K. Gupta, Inorg. Chem., 2015, 54, 2616–2625 CrossRef CAS PubMed.
- N. K. Sahu, N. Shanta Singh, R. S. Ningthoujam and D. Bahadur, ACS Photonics, 2014, 1, 337–346 CrossRef CAS.
- E. V. Samsonova, A. V. Popov, A. S. Vanetsev, K. Keevend, E. O. Orlovskaya, V. Kiisk, S. Lange, U. Joost, K. Kaldvee, U. Maeorg, N. A. Glushkov, A. V. Ryabova, I. Sildos, V. V. Osiko, R. Steiner, V. B. Loschenov and Y. V. Orlovskii, Phys. Chem. Chem. Phys., 2014, 16, 26806–26815 RSC.
- S. Rodriguez-Liviano, F. J. Aparicio, T. C. Rojas, A. B. Hungría, L. E. Chinchilla and M. Ocaña, Cryst. Growth Des., 2012, 12, 635–645 CAS.
- G. A. Kumar, N. R. Balli, M. Kailasnath, L. C. Mimun, C. Dannangoda, K. S. Martirosyan, C. Santhosh and D. K. Sardar, J. Alloys Compd., 2016, 672, 668–673 CrossRef CAS.
- Z. B. An, X. Z. Xiao, J. Yu, D. S. Mao and G. Z. Lu, RSC Adv., 2015, 5, 52533–52542 RSC.
- J. S. Xie, Q. S. Wu, D. Zhang and Y. P. Ding, Cryst. Growth Des., 2009, 9, 3889–3897 CAS.
- Q. L. Luo, S. D. Shen, G. Z. Lu, X. Z. Xiao, D. S. Mao and Y. Q. Wang, J. Mater. Chem., 2009, 19, 8079–8085 RSC.
- A. K. Parchur, A. I. Prasad, S. B. Rai, R. Tewari, R. K. Sahu, G. S. Okram, R. A. Singh and R. S. Ningthoujam, AIP Adv., 2012, 2, 032119 CrossRef.
- R. S. Ningthoujam, A. Sharma, K. S. Sharma, K. C. Barick, P. A. Hassan and R. K. Vatsa, RSC Adv., 2015, 5, 68234–68242 RSC.
- M. L. Zhao, G. S. Li, L. P. Li, L. S. Yang and J. Zheng, Cryst. Growth Des., 2012, 12, 3983–3991 CAS.
- M. N. Luwang, R. S. Ningthoujam, Jagannath, S. K. Srivastava and R. K. Vatsa, J. Am. Chem. Soc., 2009, 132, 2759–2768 CrossRef PubMed.
- M. N. Luwang, R. S. Ningthoujam, S. K. Srivastava and R. K. Vatsa, J. Am. Chem. Soc., 2011, 133, 2998–3004 CrossRef CAS PubMed.
- C. Zollfrank, H. Scheel, S. Brungs and P. Greil, Cryst. Growth Des., 2008, 3, 766–770 Search PubMed.
- Q. Y. Zhang, H. Tian, N. Li, M. D. Chen and F. Teng, CrystEngComm, 2014, 16, 8334–8339 RSC.
- A. S. Vanetsev, E. V. Samsonova, O. M. Gaitko, K. Keevend, A. V. Popov, U. Mäeorg, H. Mändar, I. Sildos and Y. V. Orlovskii, J. Alloys Compd., 2015, 639, 415–421 CrossRef CAS.
- A. K. Parchur, R. S. Ningthoujam, S. B. Rai, G. S. Okram, R. A. Singh, M. Tyagi, S. C. Gadkari, R. Tewari and R. K. Vatsa, Dalton Trans., 2011, 40, 7595–7601 RSC.
- L. L. Li, J. J. Zhang, W. W. Zi, S. C. Gan, G. J. Ji, H. F. Zou and X. C. Xu, Solid State Sci., 2014, 29, 58–65 CrossRef CAS.
- Y. L. Liu, H. L. Xiong, N. N. Zhang, Z. H. Leng, R. Q. Li and S. C. Gan, J. Alloys Compd., 2015, 653, 126–134 CrossRef CAS.
- X. W. Zhang, Z. Zhao, X. Zhang, A. Marathe, D. B. Cordes, B. Weeks and J. Chaudhuri, J. Mater. Chem. C, 2013, 1, 7202–7207 RSC.
- H. X. Guan, Y. H. Song, K. Y. Zheng, Y. Sheng and H. F. Zou, Phys. Chem. Chem. Phys., 2016, 18, 13861–13873 RSC.
- X. G. Zhang, L. Y. Zhou, Q. Pang, J. X. Shi and M. L. Gong, J. Phys. Chem. C, 2014, 118, 7591–7598 CAS.
- M. J. Xu, L. X. Wang, D. Z. Jia and H. Y. Zhao, Phys. Chem. Chem. Phys., 2015, 17, 28802–28808 RSC.
- K. Li, Y. Zhang, X. J. Li, M. M. Shang, H. Z. Lian and J. Lin, Phys. Chem. Chem. Phys., 2015, 17, 4283–4292 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra22913a |
| This journal is © The Royal Society of Chemistry 2016 |






