Hierarchically nanostructured MnO2 electrodes for pseudocapacitor application†
Ilhwan Ryua,
Green Kima,
Hajin Yoona,
Sang Jung Ahnb and
Sanggyu Yim*a
aDepartment of Chemistry, Kookmin University, Seoul 136-702, South Korea. E-mail: sgyim@kookmin.ac.kr; Tel: +82-2-910-4734
bKorea Research Institute of Standard and Science, Daejeon 305-340, South Korea
First published on 24th October 2016
Abstract
The supercapacitive properties of hierarchically nanostructured manganese oxide (MnO2) electrodes were investigated. The hierarchical MnO2 nanostructures consisting of nanowires in a small length scale and inverse-opal nanostructures in a large length scale were successfully fabricated using a combination of colloidal nanosphere lithography and cyclic voltammetric deposition. A maximum specific capacitance, Csp, of 714 F g−1 at the scan rate of 10 mV s−1 was obtained, which was significantly larger than 556 F g−1 observed for the MnO2 electrode with simple nanowire structures. The enhancement of the Csp value is attributed to the lower charge-transfer and diffusive resistance of the hierarchical nanostructures, which was estimated by electrochemical impedance measurements. The other capacitive properties were also improved considerably. The Csp retention for the hierarchical nanostructure electrode after 5000 charge–discharge cycles was 75.4%, and the voltammetric response at high scan rates was 1.8 times larger than that for the simple nanowire electrode.
Introduction
Supercapacitors are attracting growing attention for their wide range of potential applications in portable electronic equipment, hybrid vehicles and cellular devices due to their extraordinarily high energy density and capacitance.1–3 In particular, pseudocapacitors which use fast surface faradic charge-transfer reactions recently have attracted considerable interest because they exert superior capacitive performances compared to other types of supercapacitors, such as electrochemical double layer capacitors (EDLCs).3,4 Typical electrode materials used for pseudocapacitors are transition metal oxides and conductive polymers.4–6 Since Lee et al. first reported on the application of manganese oxide (MnO2) to supercapacitors,7 MnO2 has been widely studied as a promising electrode material due to its high theoretical capacitance, environmentally friendly characteristics and low price. For the charge storage of MnO2, two mechanisms were proposed, both involving a redox reaction between the III and IV oxidation states of Mn. One is based on the intercalation and deintercalation of electrolyte cations (C+) in the bulk (eqn (1)),8 and the other involves the surface adsorption of the cations on MnO2 (eqn (2)).9| MnO2 + C+ + e− ⇄ MnOOC | (1) |
| (MnO2)surface + C+ + e− ⇄ (MnO2−C+)surface | (2) |
In this work, we successfully fabricated hierarchically nanostructured MnO2 electrodes which can exploit the synergetic joining of two different length scales. The hierarchical nanostructures were formed by electrodeposition of MnO2 nanowires with dimension of a few nanometers onto substrates nanopatterned by colloidal nanosphere lithography with dimensions of several hundred nanometers. The nanostructure with larger length scale improves the electronic/ionic conductivity of MnO2 by reducing the average thickness of the MnO2 layer at equal deposition weight. The nanowire structure with smaller length scale provides large surface area and short diffusion paths for ions. The specific capacitance of this hierarchically nanostructured MnO2 electrode reached 714 F g−1 at the scan rate of 10 mV s−1 without using any additive carbon materials.
Experimental
Fabrication of hierarchically and simply nanostructured MnO2 electrodes
First, two-dimensionally (2D) close-packed polystyrene (PS) nanospheres with diameter of 580 nm (Interfacial Dynamics Co.) were transferred onto thoroughly cleaned fluorine-doped tin oxide (FTO)-coated glass substrate (FTO thickness of 1 μm, resistance of 7 Ω □−1) by a scooping transfer technique25 for fabricating periodically arrayed nanostructures. The electrodeposition of MnO2 was performed in a three-electrode system in which MnO2-deposited FTO substrate was used as working electrode, a platinum plate as counter electrode and Ag/AgCl (in saturated KCl aqueous solution) as reference electrode. The cyclic voltammetric technique was used to deposit MnO2 nanowires on the PS-coated FTO substrates by cycling the potential between 0.4 and 1.3 V in 5 mM aqueous manganese sulfate monohydrate (MnSO4·H2O, ≥ 99%, Sigma-Aldrich) solution at the scan rate of 30 mV s−1 with 0.1 M sodium sulfate (Na2SO4, ≥ 99%, Sigma-Aldrich) as supporting electrolyte and 0.01 wt% of sodium dodecyl sulfate (SDS, ≥ 99%, Sigma-Aldrich) as wetting agent. The PS nanospheres were then removed by immersing the electrodes into tetrahydrofuran (THF, ≥ 99%, Daejung Chemical and Metals Co. Ltd.) solution under stirring for 12 h. For comparison, simple nanostructures of MnO2 films were also fabricated using the same electrodeposition procedure on bare FTO-coated glass substrates without nanopatterning. After deposition, the electrodes were washed with ethanol and distilled water, followed by annealing at 60 °C for 2 h to remove residues and enhance their capacitive performance. The weight of the deposited MnO2 was determined by a quartz crystal microbalance (QCM, Stanford Research Systems QCM200).Characterization
The crystalline structure and electron binding energies of the electrodeposited MnO2 were confirmed by X-ray diffraction (XRD, Philips PW1827) and X-ray photoelectron spectroscopy (XPS, K-alpha, Thermo UK), respectively. The results (Fig. S1†) are in agreement with previously reported MnO2 nanowires.26 The structure and surface morphology of the MnO2 electrodes were characterized by field emission scanning electron microscopy (FE-SEM, JEOL JSM-7410F, JEOL Ltd.) and high-resolution transmission electron microscopy (HRTEM, JEM-2100F, JEOL Ltd.). The electrochemical properties were evaluated in 1 M aqueous Na2SO4 solution at room temperature using a cyclic voltammeter (ZIVE SP2, WonATech). The cyclic voltammetry (CV) was measured over the potential range from 0.0 to 1.0 V at scan rates of 10–200 mV s−1, and the electrochemical impedance spectroscopy (EIS) was measured over the frequency range from 10−1 to 105 Hz at open-circuit potential with an amplitude of 10 mV.Results and discussion
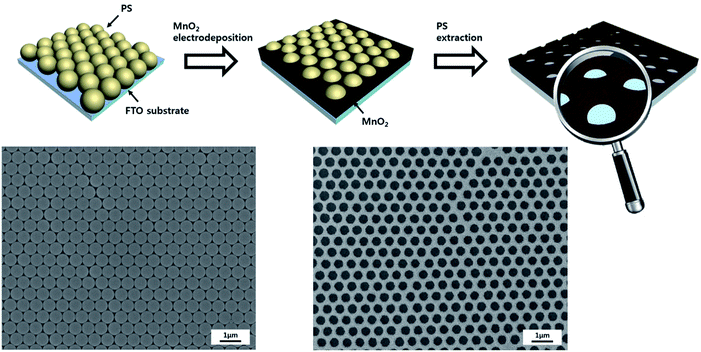
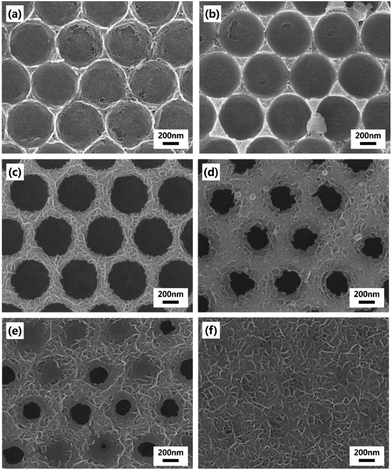
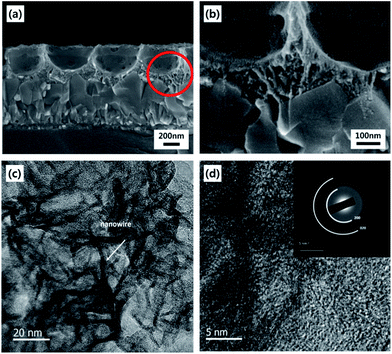
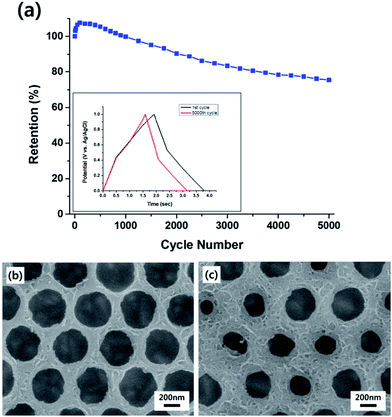
The basic strategy to fabricate hierarchical MnO2 nanostructures is illustrated in Fig. 1. First, close-packed 2D array of PS nanospheres was prepared on FTO-coated glass substrates. Then, a thin layer of MnO2 nanowires was deposited on the PS nanosphere-covered surface using the cyclic voltammetric deposition method. Finally, the PS nanospheres were extracted by the THF treatment. The FE-SEM images below the schematic illustration in Fig. 1 show that the periodically arrayed nanostructure was successfully fabricated on a large area of the substrate surface. The morphological evolution of the surfaces as a function of the number of MnO2 deposition cycles is shown in Fig. 2. MnO2 nanowires with a few nanometers in width formed on the inverse-opal nanostructures with dimension of 580 nm between the nearest-neighbour holes are clearly observed. The area of the surface covered with MnO2 nanowires gradually increased as the number of deposition cycles increased. The MnO2 layer seemed to reach the half-height of the PS nanospheres at around 15 deposition cycles (Fig. 2c). After more than 20 cycles (Fig. 2d–f), it was observed that the PS-removed spaces were gradually covered with MnO2 nanowire layers. The formation of MnO2 nanowires was more closely observed by cross-sectional FE-SEM (Fig. 3a and b) and TEM/HRTEM (Fig. 3c and d) images. The lattice fringes in Fig. 3d and SAED pattern (inset of Fig. 3d) reveal the formation of birnessite-type MnO2 polycrystalline nanowires.26 The deposit weight of the MnO2 layer as measured by QCM was almost proportional to the number of electrodeposition cycles (Fig. S2†), reaching approximately 300 μg after the 25 cycles. For comparison, the MnO2 nanowires were also electrodeposited on bare FTO-coated glass substrates, which will be denoted as simple nanostructures in contrast to the hierarchical nanostructures. The surface morphologies of these simple nanostructures after various numbers of deposition cycles were also characterized by FE-SEM as shown in Fig. 4. At 15 cycles (Fig. 4c), it is observed that almost the whole surface was covered with MnO2 nanowires. The FE-SEM image of the whisker-like nanowires became clearer at 20 cycles of deposition (Fig. 4d). After 25 cycles, the nanowires became shorter and more slender, as shown in Fig. 4e and f. In addition, some cracks on the electrode surface which are probably caused by detachment of MnO2 layers from the substrate were observed. The number and size of these cracks increased as the electrodeposition proceeded.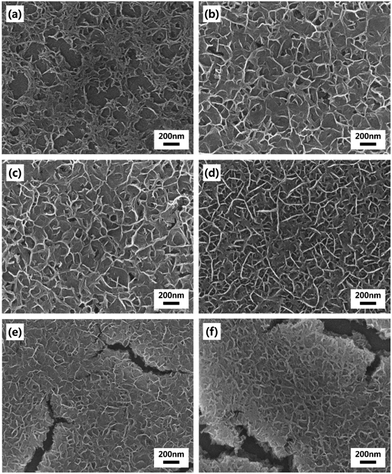 | ||
| Fig. 4 FE-SEM images of simple MnO2 nanostructures electrodeposited on bare FTO substrates. The number of electrodeposition cycles is (a) 5, (b) 10, (c) 15, (d) 20, (e) 25 and (f) 30. | ||
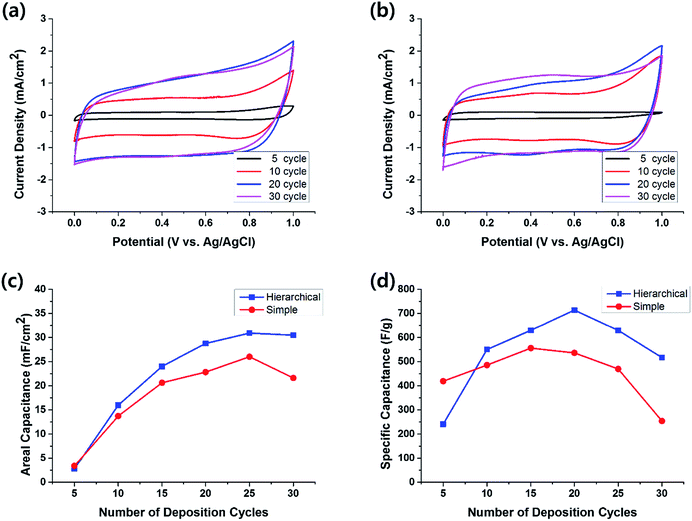
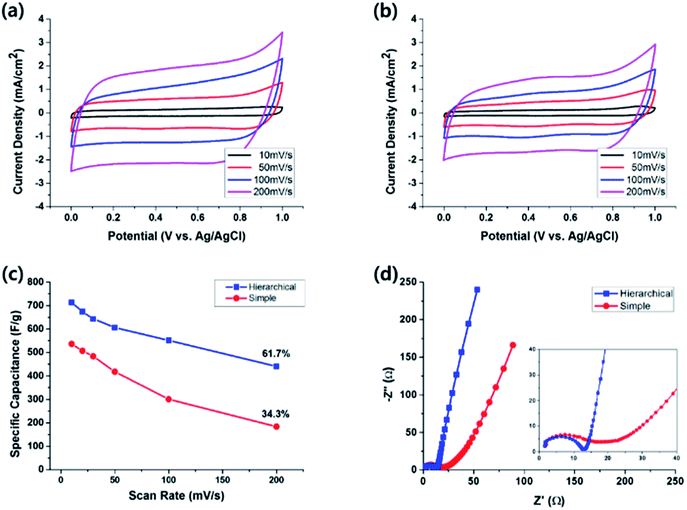
The electrochemical performance of half-cell supercapacitors based on the hierarchical and simple MnO2 nanostructures was investigated by cyclic voltammetry (CV) measured in 1 M aqueous Na2SO4 solution within the potential range from 0.0 to 1.0 V. The typical cyclic voltammograms measured at the scan rate of 100 mV s−1 for the electrodes with hierarchical and simple nanostructures are shown in Fig. 5a and b, respectively. The almost symmetric CV curves indicate the reversibility of the redox transition of MnO2. In the case of simple nanostructure electrodes, the areal capacitance was easily saturated as the MnO2 deposition preceded, or even decreased after 25 deposition cycles (red circles in Fig. 5c). This is probably because the bottom part MnO2 molecules of the thick film become electrochemically difficult to be accessible to the electrolyte. The cracks observed in Fig. 4 may also contribute to the decrease of the capacitive performance for thick electrodes. In contrast, for the cells with hierarchical MnO2 nanostructures, the increment of the areal capacitance with the number of deposition cycles (blue squares in Fig. 5c) was larger than that of the cells with simple nanostructures. At small number of deposition cycles, the areal capacitance of the cells with hierarchical nanostructure is similar to that of the cells with simple nanostructure. For example, after 5 deposition cycles, the areal capacitance for the cells with hierarchical and simple nanostructures is 2.8 mF cm−2 and 3.4 mF cm−2, respectively. However, the areal capacitance of the cells with hierarchical nanostructures increased rapidly as the deposition proceeded and exceeded that of the simple nanostructure cells after 10 deposition cycles. Notably, the hierarchical nanostructures retarded the saturation of the areal capacitance, as compared to the simple nanostructures, indicating that the benefits of nanostructures, i.e. enhancement in electrolytic accessibility, are more effectively retained for the MnO2 electrode grown on the arrayed pattern.
The specific capacitance, Csp, was also calculated by the following equation
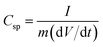 | (3) |
Conclusions
The MnO2 electrode with hierarchical nanostructures was successfully fabricated by electrodepositing MnO2 nanowires onto FTO substrates covered with periodically arrayed PS nanospheres, followed by PS extraction. The hierarchical structure consisted of inverse-opal nanostructures with dimension of a few hundred nanometers, e.g. 580 nm in this work, and nanowires with width of a few nanometers. The specific capacitance of the hierarchically nanostructured MnO2 electrode reached 714 F g−1 at the scan rate of 10 mV s−1, which is significantly larger than the maximum Csp value for the MnO2 electrode with simple nanowire structures of 556 F g−1. This enhancement of the specific capacitance without using any supportive material, such as CNTs and/or graphene, could be attributed to the synergetic effect of the two different length scales, i.e. reduced MnO2 thickness and consequently improved electronic/ionic conductivity owing to the large length-scale of the inverse-opal nanostructures, and increased surface area and consequently shortened diffusion paths for ions owing to the small length-scale of the nanowire structures. The specific capacitance of the hierarchical nanostructures retained 61.7% at the rapid scan rate of 200 mV s−1, which is significantly larger than the retention of 34.3% observed for the simple nanostructures at the same scan rate. In addition to the increased surface area, the EIS measurements confirmed that the arrayed nanopatterns lowered the charge transfer and diffusive resistance of the electrode, which also contributed to the improvement of the capacitive properties, i.e. large capacitance and rapid response. Moreover, the Csp retention of the hierarchically nanostructured electrode after 5000 charge–discharge cycles was 75.4%. All the results presented here demonstrate the benefits of the hierarchical MnO2 nanostructures for the application to pseudocapacitor electrodes.Acknowledgements
This work was supported by National Research Foundation of Korea (NRF) Grant (No. 2016R1A5A1012966, NRF-2013R1A1A2A10012336, and No. 2011-0030233) funded by the Korean Government.Notes and references
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797 RSC.
- J. Yan, Q. Wang, T. Wei and Z. Fan, Adv. Energy Mater., 2014, 4, 1300816 CrossRef.
- L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520 RSC.
- R. Liu, J. Duay and S. B. Lee, Chem. Commun., 2011, 47, 1384 RSC.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245 CrossRef CAS PubMed.
- R. Liu, J. Duay, T. Lane and S. B. Lee, Phys. Chem. Chem. Phys., 2010, 12, 4309 RSC.
- H. Y. Lee, V. Manivannan and J. B. Goodenough, C. R. Acad. Sci., Ser. IIc: Chim., 1999, 2, 565 CrossRef CAS.
- S. C. Pang, M. A. Anderson and T. W. Chapman, J. Electrochem. Soc., 2000, 147, 444 CrossRef CAS.
- H. Y. Lee and J. B. Goodenough, J. Solid State Chem., 1999, 144, 220 CrossRef CAS.
- M. Toupin, T. Brousse and D. Belanger, Chem. Mater., 2004, 16, 3184 CrossRef CAS.
- S. Liu, S. Sun and X.-Z. You, Nanoscale, 2014, 6, 2037 RSC.
- S. Devaraj and N. Munichandraiah, J. Electrochem. Soc., 2007, 154, A901 CrossRef CAS.
- M.-S. Wu, Appl. Phys. Lett., 2005, 87, 153102 CrossRef.
- J. Wei, N. Nagarajan and I. Zhitomirsky, J. Mater. Process. Technol., 2007, 186, 356 CrossRef CAS.
- W. Xiao, H. Xia, J. Y. H. Fuh and L. Lu, J. Power Sources, 2009, 193, 935 CrossRef CAS.
- V. Subramanian, H. Zhu, R. Vajtai, P. M. Ajayan and B. Wei, J. Phys. Chem. B, 2005, 109, 20207 CrossRef CAS PubMed.
- S. W. Lee, J. Kim, P. T. Hammond and Y. Shao-Horn, ACS Nano, 2010, 4, 3889 CrossRef CAS PubMed.
- R. Liu, J. Duay and S. B. Lee, ACS Nano, 2010, 4, 4299 CrossRef CAS PubMed.
- H. Xia, J. Feng, H. Wang, M. O. Lai and L. Lu, J. Power Sources, 2010, 195, 4410 CrossRef CAS.
- Jaidev, R. I. Jafri, A. K. Mishra and S. Ramaprabhu, J. Mater. Chem., 2011, 21, 17601 RSC.
- X. Dong, W. Shen, J. Gu, L. Xiong, Y. Zhu, H. Li and J. Shi, J. Phys. Chem. B, 2006, 110, 6015 CrossRef CAS PubMed.
- K.-W. Nam, C.-W. Lee, X.-Q. Yang, B. W. Cho, W.-S. Yoon and K.-B. Kim, J. Power Sources, 2009, 188, 323 CrossRef CAS.
- J. Yan, Z. Fan, T. Wei, W. Qian, M. Zhang and F. Wei, Carbon, 2010, 48, 3825 CrossRef CAS.
- G. Yu, L. Hu, M. Vosgueritchian, H. Wang, X. Xie, J. R. McDonough, X. Cui, Y. Cui and Z. Bao, Nano Lett., 2011, 11, 2905 CrossRef CAS PubMed.
- J. R. Oh, J. H. Moon, S. Yoon, C. R. Park and Y. R. Do, J. Mater. Chem., 2011, 21, 14167 RSC.
- D. P. Dubal, D. Aradilla, G. Bidan, P. Gentile, T. J. S. Schubert, J. Wimberg, S. Sadki and P. Gomez-Romero, Sci. Rep., 2015, 5, 9771 CrossRef CAS PubMed.
- C. D. Lokhande, D. P. Dubal and O. S. Joo, Curr. Appl. Phys., 2011, 11, 255 CrossRef.
- L. Wang, H. Ji, F. Zhu, Z. Chen, Y. Yang, X. Jiang, J. Pinto and G. Yang, Nanoscale, 2013, 5, 7613 RSC.
- J.-W. Wang, Y. Chen and B.-Z. Chen, J. Electrochem. Soc., 2015, 162, A1654 CrossRef CAS.
- E. Barsoukov and J. R. Macdonald, Impedance Spectroscopy: Theory, Experiment, and Applications, Wiley Interscience, 2005 Search PubMed.
- Z. Lei, J. Zhang and X. S. Zhao, J. Mater. Chem., 2012, 22, 153 RSC.
- J. Zhi, O. Reiser and F. Huang, ACS Appl. Mater. Interfaces, 2016, 8, 8452 CAS.
- H. Jiang, L. Yang, C. Li, C. Yan, P. S. Lee and J. Ma, Energy Environ. Sci., 2011, 4, 1813 CAS.
- F. Ataherian and N.-L. Wu, J. Electrochem. Soc., 2011, 158, A422 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Powder XRD pattern of electrodeposited MnO2 thin films, Bode |Z| and Bode angle plots of the hierarchical and simple MnO2 nanostructure electrodes, a photograph of a red LED lightened by three asymmetric supercapacitor units connected in series. See DOI: 10.1039/c6ra22841k |
| This journal is © The Royal Society of Chemistry 2016 |