Experimental research on the thermal decomposition of pentafluoroethane (HFC-125) extinguishing agent with n-heptane/air pool fire in confined space
Wang Tingab,
Qin Kuanga,
Wang Huaa and
Pan Renming*a
aNanjing University of Science and Technology, Nanjing, 210094, China. E-mail: panrenming@njust.edu.cn
bJiangsu Police Institute, Nanjing, 210031, China
First published on 21st November 2016
Abstract
To discuss the production law of hazardous gases (e.g. hydrogen fluoride) during the interaction between a pentafluoroethane extinguishing agent and an n-heptane flame, we designed a simulation of experimental total flooding conditions that is highly similar to the actual total flooding automatic extinguishing conditions. In this study, a qualitative and quantitative analysis on the thermolysis gaseous products was performed through GC-MS and ion chromatography (IC) by studying the thermal decomposition characteristics of the interaction between a pentafluoroethane extinguishing agent and an n-heptane flame in a 1 m3 fire-smothering room under different fire sizes and interaction times. Research results demonstrated that the thermolysis gaseous products of the pentafluoroethane extinguishing agent and n-heptane flame include hydrogen fluoride (HF) and other organic gases, such as CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2 and CF3CH2CF3. The HF output was proportional to the fire size and interaction time. When the interaction time was controlled within 10 s, production of hazardous gases could be effectively reduced.
CF2 and CF3CH2CF3. The HF output was proportional to the fire size and interaction time. When the interaction time was controlled within 10 s, production of hazardous gases could be effectively reduced.
1. Introduction
In firefighting scenes, rescue workers, escapees and other living bodies may come in direct contact with the tail gases generated from the interaction between an extinguishing agent and flames.1–4 Therefore, understanding the formation, composition and toxicity features of these gases is important to provide significant guidance to avoid similar smoke damage in further applications.1,5,6 Existing reports on the characteristics of tail gases generated during the firing of a new fluorine-containing extinguishing agent mainly focus on trifluoromethane,7,8 heptafluoropropane,9,10 C6-fluoroketone,11 and pentafluoroethane (HFC-125),12 especially when the extinguishing agents come in close contact with the common flame (B-class)/air conditions.HFC-125, mainly used as a refrigerant for a long time, has been chosen as a new extinguishing agent to replace halon in the developed countries like Europe and America because of its characteristics of being colourless, almost tasteless, non-conductivity, corrosion resistance, easy storage and having a high extinguishing efficiency.12–14 Moreover, as zero value for ozone-depletion potential (ODP) and no residue after fire extinguishing, HFC-125 has captured wide attention with its ODP, fire extinguishing convenience and being economical, high clean class after fire extinguishing in the field of aircraft fire protection.15,16 Its basic physical and chemical properties, extinguishing efficiency, and engineering applications has been well studied in the previous reports.17–19 However, only few research studies on the tail gases generating from the interaction between HFC-125 and a B-class flame have been reported to date. Correspondingly, there are a few research studies that have discussed the composition of tail gas generating from the combustion of HFC-125. Daniel et al.20 studied a HFC-125 extinguishing agent to inhibit a CH4–O2–air low-pressure premixed flame and detected a series of combustion components by tunable diode laser absorption spectroscopy (TDLAS). Andersson et al.21 analyzed the thermolysis products of the interaction between HFC-125 and the dimethylmethane diffusion flame by using Fourier transform infrared spectroscopy (FT-IR) and ion chromatography (IC), detecting the production of HF and fluorophosgene. Under a low material supply rate, almost all fluorine elements were converted into HF. The fluorophosgene output was positively correlated with the material supply rate, whereas the HF output was negatively correlated. Recently, Linteris et al.12 estimated HF production by equilibrium calculations when using pentafluoroethane to inhibit the methane–air flame and conducted the extinguishing experiments using different scales of a cup-shaped burner. Other well published studies22,23 also presented the influences of HFC 125 with bromide on the extinguishing process and gave the primary formulation of tail gases.
To summarize, in the existing reports, the researchers have detected toxicants (e.g. fluorophosgene and CO) in the tail gas after the combustion of HFC-125 besides HF as well as the relationship between the production of these toxicants and reaction conditions. However, almost all of this work is mainly based on cup-like burner experiments and basically a constant flame intensity, which is regarded as significantly different from the actual firefighting under total flooding conditions. Because the flame intensity in a cup-like burner is relatively low, the flame intensity in an actual fire scene gradually declines due to the inhibition by the extinguishing agents. In actual firefighting, the extinguishing agents may repeatedly interact with flames.
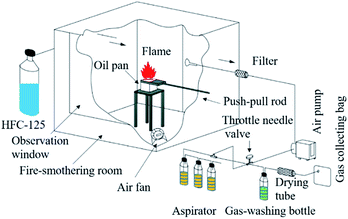
In this study, to solve this problem, we first designed a relatively sealed fire-smothering room, which ensured sufficient interaction between HFC-125 and the flame in a confined space. Since gases easily diffuse in open experimental systems, extinguishing agents in previous cup-like extinguishment experiments were carried away by the airflow after they interacted with the flame, which could not ensure a sufficient interaction time and effect. This was similar to the extinguishing conditions by gaseous extinguishing agents under the total flooding conditions and closer to the actual firefighting situations. Moreover, we also carefully designed the experimental conditions close to those in the actual situations. For offering a high flame intensity, we made an oil pool (n-heptane) on spontaneous combustion in a confined room under different working conditions without using any extinguishing agent. Even for a given HFC-125 concentration, the flame intensity on the oil pool weakened and the specific fuel consumption slowed down together with a gradual decrease in oxygen in the fire-smothering room. However, previous experiments involved igniting the fuels, and then spraying the extinguishing agent, making it difficult to maintain the appropriate concentration of the fire extinguishing agent. A high concentration of HFC-125 will cause strong flame inhibition, resulting in an inadequate interaction time and low concentration leading to a strong flame with insufficient interaction and weak signals. Therefore, to study the toxicity features of these gases, the HFC-125 concentration in the fire-smothering room was set to 50% of the extinguishment concentration to ensure that the interaction time between HFC-125 and n-heptane flames was adequately maintained to guarantee the full interaction between the extinguishing agent and the flame.
The objective of this study was to investigate the effects of the actual interaction between the HFC-125 and the flame under different fire sizes and interaction times. Moreover, we tried to analyze the possible tail gases (e.g. HF) by using ion chromatography and GC-MS. It could provide an effective benchmarking study on the thermolysis of gases in the thermal decomposition of HFC-125 and an n-heptane/air flame. Also, it might be useful for the real application of the extinguishing agent and for reducing the risk of unintended accidental losses in the firefighting scenes.
2. Experimental section
2.1 Materials and chemicals
Pentafluoroethane (C2F5H, purity > 99.7%) was obtained from SINOCHEM LAMTIAN Inc. The combustible fuel was n-heptane. The oil pool was designed by our own laboratory. The flame model of the oil pool was of three sizes: (1) 5.0 cm × 5.0 cm × 8.0 cm, (2) 7.5 cm × 7.5 cm × 8.0 cm and (3) 10.0 cm × 10.0 cm × 8.0 cm. The loaded 3.0 mm thick n-heptane liquid level was 5.0 mm below the oil pool opening and the oil pool bottom was paved with clean water. Glass rotameter (LZB-6) was purchased from Shanghai Tianchuan instrument and meter plant. A soap film flowmeter (LZM-1) was purchased from Shanghai Hongyu Institute of environmental protection application.2.2 Experimental preparation
2.3 Experimental characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 100 μL, respectively. The MS conditions include EI ionization way, 70 eV electron multiplier pressure, 230 °C ion source temperature and a m/z 10–650 scan range.
1 and 100 μL, respectively. The MS conditions include EI ionization way, 70 eV electron multiplier pressure, 230 °C ion source temperature and a m/z 10–650 scan range.3. Results and discussion
3.1 Oil pool fire flame test
For a typical class B flame, various types and theoretical models of n-heptane flames have been studied for more than thirty years.24–26 Although some studies exist in the literature27 that have investigated the relationship between the structure of the n-heptane flames and certain extinguishing agents, it is still necessary for us (1) to precisely determine the rate of heat released from the pool-fire flames and (2) eliminate the self-inhibition effect caused by the decrease in the oxygen concentration in a confined space with different oil pool sizes (5, 7.5, 10 cm) by weighing the mass of the given n-heptane. Fig. 2 gives the relationships between the burning time and the oil mass (gram) of n-heptane in an oil pool with different pool sizes (Fig. 2a). The results show that the oil mass of n-heptane gradually decreased with an increase in the burning time and the burning time was longer than 200 s, which indicates that the free flame of the pool fire cannot be inhibited by a decrease in the oxygen concertation at a certain period of time, and the durations of burning are as short as 273 s (Fig. 2b, 5 cm), 267 s (Fig. 2c, 7.5 cm) and 223 s (Fig. 2d, 10 cm). Note that as the concentration of oxygen decreases in a confined space, the burning time decreases to 249.5 s, 213.6 s and 196.9 s with an increase in the pool size. And all three oil pan do not be lighted again. | ||
| Fig. 2 Burning time of the oil pool fire with different pool sizes (5, 7.5, and 10 cm) in a confined space. | ||
After customization of the rate of heat released from the flames and calibration of the HFC-125 concentration, the interaction time is the key step for studying the interaction between HFC-125 and the n-heptane/air pool fire in the confined fire-smothering room. The rate of the heat released can obtained by the following formula,
| Q = χ × m × ΔH | (1) |
3.2 Concentration calibration of HFC-125
For researching the flame interaction of the HFC-125 extinguishing agent of n-heptane/air pool fire in a confined space, in addition to the rate of heat released, we should also determine the concentration of HFC-125. Table 1 shows the details of the actual value of HFC-125 repeated three times for the precise determinations. Furthermore, we also fit the curve of the actual value of HFC-125 to adjust the amount of the extinguishing agent as a linear fit mode by the Origin 8.0 software according to the following equation:| y = 0.6148x + 32.2 | (2) |
| Set value (L h−1) | Cva (mL) | Measuring timeb (s) | Average value (s) | Actual value (L h−1) | ||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| a Cv (calibration volume), is set according to a set value of HFC-125 concentration.b Measuring time can be determined as a single soap film flowing through a 1000 mL volume in the soap membrane flowmeter via a stopwatch. | ||||||
| 100 | 1000 | 38.77 | 38.80 | 38.80 | 38.79 | 92.80 |
| 200 | 1000 | 23.12 | 23.13 | 23.12 | 23.12 | 155.71 |
| 300 | 1000 | 16.49 | 16.50 | 16.51 | 16.50 | 218.18 |
| 400 | 1000 | 13.31 | 13.30 | 13.30 | 13.30 | 270.68 |
In principle, we can obtain the amount of HFC-125 at any time. For maintaining an adequate interaction time to guarantee a full interaction between HFC-125 and the flame, the HFC-125 concentration in the fire-smothering room was set to 50% of the extinguishment concentration to ensure an adequate interaction time between HFC-125 and the n-heptane/air flames. The extinguishment concentration of HFC-125 was 9.3% according to ISO14520-8,28 and thus the 50% value of its extinguishment concentration was 4.65%. Therefore, for a given volume of the fire-smothering room (1000 L here), it required 46.5 L (or 248.2 g) of HFC-125 under standard conditions. In this experiment, 50% (corresponds to n-heptane fuel) of HFC-125 should be injected into the fire-smothering room first, and then the n-heptane fuel was ignited to ensure an adequate interaction time.
3.3 Determining the interaction time of HFC-125
When the HFC-125 concentration was 50% of its extinguishment concentration, the flame intensity on the oil pool weakened and the specific fuel consumption slowed down, which would surely prolong the spontaneous firing. This result agrees well with that of an another reported study.28 The flame intensity gradually weakened since oxygen in the fire-smothering room was gradually consumed. Therefore, the smoke that was generated in the first 30 s was collected for tail gas analysis. Specifically, the interaction time between HFC-125 and the n-heptane flames was after the inhibition of the fire by HFC-125 controlled within 30 s. It was speculated that during the interaction of HFC-125 and the n-heptane/air flame, the power of the flame at early times slightly changed. This means that the interaction time between HFC-125 and the n-heptane flames was controlled within 30 s.3.4 HF content test
HF is a highly toxic substance and it has strong stimulus and corrosion effects on the respiratory mucous membranes and skin.29 Inhalation of high concentrations of HF will cause pneumonia, tracheitis, toxic effects to the whole body and even fluorosis of bone.30 Therefore, determining the HF content in the thermolysis gaseous products after the interaction between HFC-125 and an n-heptane flame is conducive to determine the toxicity of the products. A pH paper test showed that the water solution of thermolysis gaseous products is strongly acidic, indicating the possibility of HF generation. It can also be inferred from the related studies that the gaseous products from the thermolysis of the reaction between HFC-125 and the flame may include HF.31 Herein, the HF content was measured by a three-way valve. Before the test, 1000 mg L−1 of fluorine ion mother liquor was prepared using NaF, and then it was diluted to 5, 10, 15, 20 and 25 mg L−1 of fluorine ion standard liquids using aspirator bottles filled with 300 mL deionized water to absorb the thermolysis gases for 30 min.| Oil pool size (cm) | Soap film volume (L) | Actual value (L min−1) | Time (min) | Gas volume (L) | Sample 1 (mg L−1) | Sample 2 (mg L−1) | Sample 3 (mg L−1) | Total (mg L−1) | Sum of HF (mg) | HF of 1 m3 (mg) |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 × 5 | 0.8 | 3.78 | 15 | 56.69 | 22.22 | 1.09 | 0 | 23.31 | 6.99 | 123.37 |
| 7.5 × 7.5 | 1 | 3.85 | 15 | 57.69 | 20.71 | 5.57 | 0 | 26.28 | 7.88 | 136.67 |
| 10 × 10 | 1 | 5.51 | 10 | 55.10 | 65.06 | 12.98 | 0 | 78.04 | 23.41 | 424.89 |
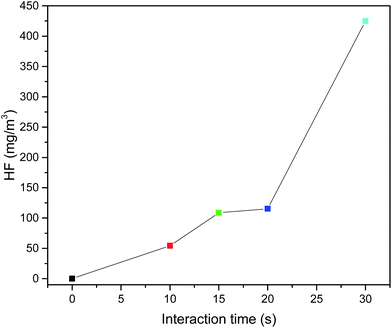
The relationship between the oil poor fire size and HF content in the thermolysis gaseous products when the interaction time is fixed at 30 s is shown in Fig. 3. It is clear from Fig. 3 that for a given interaction time, HF is also detected in the flame interaction of HFC-125 and the n-heptane/air pool fire in a confined space. The HF content increases as the pool size increases. The HF content reaches to 123.3 mg m−3, when the fire size is 0.90 kW (5 × 5 cm). HF production slightly increased within 2.5 kW (7.5 × 7.5 cm) and increased to the highest of 424.9 mg m−3 as the fire size increased to 4.79 kW (10 × 10 cm). In this situation, due to the existing 50% concentration of HFC-125, the strong flame intensity is regarded as a full interaction with the flame until we pushed the push–pull rod off. This means that the experimental apparatus designed in this study can be considered to be the real situation in firefighting scenes by overcoming the weak fire flame intensity and gradually decreasing the flame intensity. Of course, in the real situation, HF can be diffused during this time period. As a result, we can determine the highest content of HF, which is a key for application and formulation of Material Safety Data Sheets (MSDS).
| Time (s) | Flowing time (s) | Actual value (L min−1) | Measuring time (min) | Gas volume (L) | Sample 1 (mg L−1) | Sample 2 (mg L−1) | Sample 3 (mg L−1) | Total (mg L−1) | Sum of HF (mg) | HF of 1 m3 (mg) |
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 12.9 | 4.65 | 15 | 70.56 | 11.38 | 1.42 | 0 | 12.80 | 3.84 | 54.43 |
| 15 | 12.7 | 4.72 | 15 | 70.87 | 23.42 | 2.24 | 0 | 25.68 | 7.70 | 108.65 |
| 20 | 16.2 | 3.70 | 15 | 55.56 | 20.51 | 0.88 | 0 | 21.39 | 6.42 | 115.48 |
| 30 | 9.8 | 5.51 | 10 | 55.10 | 65.06 | 12.98 | 0 | 78.04 | 23.41 | 424.89 |
3.5 Analysis of other organic ingredients of tail gas
A qualitative analysis of the tail gas composition was implemented and the organic gases produced after the interaction between HFC-125 and the flame under these conditions were collected for the GC-MS analysis (Fig. 5). For an accurate analysis, the parameters of the typical interaction were chosen as the highest flame intensity (4.79 kW) under the longest interaction time (30 s) and as abovementioned in Section 3.4.2, the maximum content. Contrary to the previously reported results, besides HF and the n-heptane fuel (Fig. 5a), the tail gas also includes some organic gases, such as CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2 (Fig. 5b) and CF3CH2CF3 (Fig. 5c), as shown in Table 4.
CF2 (Fig. 5b) and CF3CH2CF3 (Fig. 5c), as shown in Table 4.
 | ||
| Fig. 5 GC-MS analysis results of the organic ingredients of the tail gas generated due to the interaction between HFC-125 and the n-heptane flame: (a) C7H16, (b) CF3CHCF2 and (c) CF3CH2CF3. | ||
| Time (min) | Mass-to-charge ratio (m/z) | Products |
|---|---|---|
| 2.974 | CF2CHCF2+(113), CFCCF2+(93), CF3CH+(82), CF3+(69), CF2CH+(63), CF+(31) | CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2 CF2 |
| 4.886 | CF2CH2CF3+(133), CFCHCF3+(113), CF3CH+(82), CF3+(69), CF+(31) | CF3CH2CF3 |
Both CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2 and CF3CH2CF3 are irritating gases.33 CF3CH
CF2 and CF3CH2CF3 are irritating gases.33 CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2 irritates the eyes, respiratory system and skin, whereas short-time exposure to CF3CH2CF3 will cause headache, debilitation, dizziness, nausea, vomiting, dyspnea and pricking, and even cardiac arrhythmia, hyperspermia, coma and asphyxia.34 Although their contents are far lower than those of the inorganic gaseous products of HF, they also can cause certain damage to the human body. It is speculated that pentafluoropropene and hexafluoropropane are produced by flame interference in relatively closed total flooding conditions, repeated reaction of the extinguishing agent and flame, as well as the chemical reaction of the extinguishing agent with the intermediate products and combustion products in the flame region.
CF2 irritates the eyes, respiratory system and skin, whereas short-time exposure to CF3CH2CF3 will cause headache, debilitation, dizziness, nausea, vomiting, dyspnea and pricking, and even cardiac arrhythmia, hyperspermia, coma and asphyxia.34 Although their contents are far lower than those of the inorganic gaseous products of HF, they also can cause certain damage to the human body. It is speculated that pentafluoropropene and hexafluoropropane are produced by flame interference in relatively closed total flooding conditions, repeated reaction of the extinguishing agent and flame, as well as the chemical reaction of the extinguishing agent with the intermediate products and combustion products in the flame region.
These results also indicate that the possible reaction of HF formulation is as follows
CF3CH2CF3 → CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2 + HF↑ CF2 + HF↑ |
This possible pathway is also in agreement with that reported in a former study.35 However, compared with the thermal decomposition reaction pathway of FEC-125,31 the interaction of HFC-125 and the flame is more complex and needs to be studied in the future.
4. Conclusions
In summary, we studied the thermolysis decomposition characteristics of the interaction between HFC-125 and n-heptane in a confined space under different fire sizes (0.90 kW, 2.49 kW and 4.79 kW) and the interaction times (10 s, 15 s, 20 s and 30 s) using an ideal oil pool fire. A qualitative and quantitative analysis on the thermolysis gaseous products by combining an IC and GC-MS analytical method was performed. It was concluded that:(1) HF production from the interaction between HFC-125 and n-heptane/air flames was influenced by the fire size. With an increase in fire size, the HF content slowly increased, and then significantly accelerated. At the minimum fire size (0.90 kW), the interaction between HFC-125 and the n-heptane flames was fierce and the HF content was very high, reaching a value of 123.3 mg m−3.
(2) The interaction time can also be influenced by HF production from the interaction between HFC-125 and the n-heptane flames. As the interaction time increases, HF production slowly increases in the first 10 s of combustion, and then significantly accelerates. After 20 s of combustion, the HF content quickly increases from 115.5 mg m−3 to 424.9 mg m−3, which is the maximum content.
(3) Given a 4.79 kW fire size and 30 s interaction time, HF production reached its peak (424.9 mg m−3). The organic gaseous products from the interaction between HFC-125 and the n-heptane flames include CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2 and CF3CH2CF3, which are irritants and may cause certain damage to the person at the site of the fire.
CF2 and CF3CH2CF3, which are irritants and may cause certain damage to the person at the site of the fire.
Smoke from the combustion of HFC-125 contains toxic and harmful substances (e.g. HF). Production of these toxic and harmful substances is positively related to the fire size and interaction time. Since it is difficult to control the fire size in actual firefighting, the interaction time between HFC-125 and the flame shall be strictly controlled within 10 s to inhibit the production of HF and other harmful gases. This could effectively control the potential risks related with the use of HFC-125.
Acknowledgements
The work was supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant No. 13KJD620001), the project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the collaborative Innovation Centre of Social Safety Science and Technology.Notes and references
- J. S. Bieback, US Pat., 5990793, 1999.
- T.-N. Guo and Z.-M. Fu, Fire Saf. J., 2007, 42, 171–182 CrossRef.
- F. Amon, A. Hamins, N. Bryner and J. Rowe, Fire Saf. J., 2008, 43, 541–550 CrossRef.
- W.-T. Tsai, Chemosphere, 2005, 61, 1539–1547 CrossRef CAS PubMed.
- J. S. Nimitz, R. E. Tapscott and S. R. Skaggs, US Pat., 5135054, 1992.
- D. E. Olander, US Pat., 5861106, 1999.
- B. Cong, F. Qi, G. Liao, K. Kuang and X. Zhou, Chin. Sci. Bull., 2005, 50, 1429–1434 CrossRef CAS.
- W. Han, E. M. Kennedy, S. K. Kundu, J. C. Mackie, A. A. Adesina and B. Z. Dlugogorski, J. Fluorine Chem., 2010, 131, 751–760 CrossRef CAS.
- B. A. Williams, D. M. L'espÉrance and J. W. Fleming, Combust. Flame, 2000, 120, 160–172 CrossRef CAS.
- X. Ni and W. Chow, Appl. Therm. Eng., 2011, 31, 3864–3870 CrossRef CAS.
- T. A. Paul, A. Kramer and M. Ingold, Switzerland Pat., EP2013/068281, 2015.
- J. L. Pagliaro, G. T. Linteris, P. B. Sunderland and P. T. Baker, Combust. Flame, 2015, 162, 41–49 CrossRef CAS.
- J. L. Pagliaro, G. T. Linteris and V. I. Babushok, Combust. Flame, 2016, 163, 54–65 CrossRef CAS.
- G. T. Linteris, V. I. Babushok, P. B. Sunderland, F. Takahashi, V. R. Katta and O. Meier, Proc. Combust. Inst., 2013, 34, 2683–2690 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- E. Edney and D. Driscoll, Int. J. Chem. Kinet., 1992, 24, 1067–1081 CrossRef CAS.
- T. Kawano, H. J. Trochimowicz, G. Malinverno and G. Rusch, Toxicol. Sci., 1995, 28, 223–231 CrossRef CAS.
- C.-c. Piao, I. Iwata and M. Noguchi, Fluid Phase Equilib., 1998, 150, 313–322 CrossRef.
- L. Wilson, W. Wilding, G. Wilson, R. Rowley, V. Felix and T. Chisolm-Carter, Fluid Phase Equilib., 1992, 80, 167–177 CrossRef CAS.
- R. G. Daniel, K. L. McNesby and A. W. Miziolek, Appl. Opt., 1996, 35, 4018–4025 CrossRef CAS PubMed.
- B. Andersson and P. Blomqvist, Fire Saf. J., 2011, 46, 104–115 CrossRef CAS.
- F. Takahashi, V. R. Katta, G. T. Linteris and O. C. Meier, Proc. Combust. Inst., 2013, 34, 2707–2717 CrossRef CAS.
- F. Takahashi, V. R. Katta, G. T. Linteris and V. I. Babushok, Proc. Combust. Inst., 2015, 35, 2741–2748 CrossRef CAS.
- M. Y. Choi, D. Frederick L and J. B. Haggard, Symp. (Int.) Combust., 1991, 23, 1597–1604 CrossRef.
- F. Tao, GOLOVITCHEV, #160, V. I., CHOMIAK, #160 and Jerzy, Self-ignition and early combustion process of N-heptane sprays under diluted air conditions: Numerical studies based on detailed chemistry, Society of Automotive Engineers, New York, NY, ETATS-UNIS, 2000.
- J. Prager, H. N. Najm, M. Valorani and D. A. Goussis, Combust. Flame, 2011, 158, 2128–2144 CrossRef CAS.
- F. Takahashi, V. R. Katta, G. T. Linteris and O. C. Meier, Proc. Combust. Inst., 2013, 34, 2707–2717 CrossRef CAS.
- BS EN 15004-4:2008 Fixed firefighting systems. Gas extinguishing systems. Physical properties and system design of gas extinguishing systems for HFC-125 extinguishant.
- A. K. Kim, J. Fire Prot. Eng., 2004, 14, 265–281 CrossRef.
- W.-T. Tsai, J. Fluorine Chem., 2007, 128, 1345–1352 CrossRef CAS.
- T. Wang, Y.-j. Hu, P. Zhang and R.-m. Pan, J. Fluorine Chem., 2016, 190, 48–55 CrossRef CAS.
- B. L. Matthews, Chemical Sensitivity: A Guide to Coping with Hypersensitivity Syndrome, Sick Building Syndrome and Other Environmental Illnesses, McFarland, 1992 Search PubMed.
- S. C. Jackson, P. R. Resnick and S. H. Swearingen, US Pat., 5910615, 1997.
- N. H. Proctor, J. P. Hughes and G. J. Hathaway, Proctor and Hughes' chemical hazards of the workplace, John Wiley & Sons, 2004 Search PubMed.
- N. L. Garland and H. Nelson, Chem. Phys. Lett., 1996, 248, 296–300 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |