A bio-hybrid material for adsorption and degradation of phenanthrene: bacteria immobilized on sawdust coated with a silica layer
Jinghua Lia,
Chuling Guo*ab,
Changjun Liaoc,
Menglu Zhangd,
Xujun Lianga,
Guining Luabe,
Chen Yangab and
Zhi Dang*abe
aSchool of Environment and Energy, South China University of Technology, Guangzhou 510006, PR China. E-mail: clguo@scut.edu.cn; chzdang@hotmail.com; Tel: +86-20-39380512 Tel: +86-20-87110198
bThe Key Lab of Pollution Control and Ecosystem Restoration in Industry Clusters, Ministry of Education, Guangzhou 510006, PR China
cDepartment of Environmental Engineering, Guangdong Polytechnic of Environmental Protection Engineering, Foshan 528216, PR China
dKey Lab of Urban Environment and Health, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, PR China
eGuangdong Provincial Engineering and Technology Research Center for Environmental Risk Prevention and Emergency Disposal, Guangzhou 510006, PR China
First published on 3rd November 2016
Abstract
Cell immobilization technology has been considered as an effective method for bioremediation of hydrocarbon-contaminated soil. However, bacteria immobilized by a single method often encounter some problems, e.g., cell leakage, cellular damage and no reproduction. In this study, a biomimetic hybrid material was constructed by pre-immobilization of bacteria on sawdust followed by coating a silica layer through vapor deposition (Silica-IC). The viability and metabolic activity of Silica-IC were investigated. Results showed that the silica layer covering the bacterial agent could significantly reduce cell leakage from sawdust without losing reproductive capacity on nutrient plates. A viability assay by SYTO9/PI in flow cytometry indicated that the proportion of live cells was decreased 30% and injured cells was increased 23.9%, while that of dead cells was still below 2.5% during storage at 4 °C for 15 days, i.e., membrane permeability of Silica-IC was increased, indicating bacterial cells in Silica-IC were able to maintain long-term storage stability and shelf life. The metabolic activity of Silica-IC toward phenanthrene (Phe) was enhanced both in liquid and soil. Phe degradation kinetics of Silica-IC in liquid medium well fitted an adsorption–degradation model, suggesting that the silica layer did not inhibit Phe diffusion. Moreover, the Phe removal percentage of Silica-IC in soil was up to 93.4% on day 2. Silica-IC in soil grew well and the growth was closely related to the residual amount of Phe. This work provides a route to develop a wide range of bio-materials for bioremediation.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous organic pollutants. It is widely accepted that biodegradation is the major process of PAHs removal. Nevertheless, PAH-contaminated soil is considered as oligotrophic and harsh habitats for microbial inocula to survive and metabolize.1 This may result from various abiotic and biotic factors in the contaminated environments,2 frequently leading to bio-augmentation unsuccessful.It has been demonstrated that cell immobilization can enhance the survival and activity of introduced microorganisms.2,3 One of the primary roles of carriers in soil is to provide protective microhabitats for cells from various environmental stresses, predation, competition and toxic substances.4,5 The soil matrix is heterogeneous, and thus the matrix created by immobilization is expected to adsorb contaminants and deliver carbon sources to inocula.6,7 Moreover, porous carriers may act as a soil-bulking agent to diffuse oxygen, water and substrates.8 Numerous carriers prepared from natural and synthetic materials have been used for microbial immobilization through different forms, primarily by adsorption onto a surface or by encapsulation in various organic or inorganic matrices.4
Cellulose-containing materials, particularly agriculture and forestry residues (e.g., sawdust, corncob powder and peanut hull powder),8,9 are commonly used for physical adsorption of cells. These materials with high water holding capacity, high volume and low weight are biocompatible, inexpensive, locally available, biodegradable and environmentally friendly. Moreover, the adsorption immobilization process is mild, quick, simple and no need for chemical additives.10 However, an obvious disadvantage is that a significant leakage of cells from materials is generally observed due to the relatively weak binding force between cells and carriers.11
It was reported that materials coated with silica gel, a membrane-forming agent, were able to improve mechanical properties of carriers, enhance adsorption abilities, reduce cell leakages and maintain cell viability.11–13 The elaboration of such bio-composites is rendered possible by recent developments of sol–gel chemistry. Actually, silica gel is another popular immobilization method, i.e., encapsulation. As a porous inorganic matrix, silica gel offers many advantages, such as superior mechanical, chemical, thermal and photochemical stability, optical transparency, tunable porosity and flexibly applicative forms (e.g., coatings, granules and shaped bulk products).14,15 Furthermore, silica is a common and abundant component of soil, making it suitable as an immobilization support.
In 1989, G. Carturan and his co-workers16 first reported that living cells encapsulated within the sol–gel derived silica matrix could maintain their long-term viability and catalytic activity. Since then, various living cells, such as bacteria, yeasts, algae, plant and animal cells,14 have already been designed for applications in bioreactors, biosensors, biofuel cells, tissue therapy and even bio-chips.17,18 However, silica sol–gel encapsulation technology entering in the bio-remediation field is more limited than one could expect from its versatility and flexibility. One possible reason is that only a few works report on its possibilities and achievements in bioremediation, and mainly focus on the adsorption of heavy metal19–23 as well as absorption or degradation of phenol15,24,25 and dyes.26–28 Another argument lies in the fact that, although sol–gel process is now performed under mild conditions at room temperature, the directly encapsulated cells by aqueous sol–gel, based on alkoxide or silicate, are usually exposed to acidic or basic environments. Furthermore, the release of ethanol/methanol or sodium ions in excess may impose significant stresses on encapsulated cells.29,30 Another limitation for bioremediation is that cells do not have enough space to divide inside the sol–gel matrix.30,31
To overcome these problems, it is feasible to pre-immobilize cells within a biocompatible natural matrix (such as sawdust) that can be further modified by silica gel.32 This hybrid material should combine the benefits of natural materials and silica gel.13,22 Furthermore, the Biosil technology, i.e., one-step vapor deposition of nanostructured silica layer on material/cell surface using silicon alkoxide vapors in a moist atmosphere,33 provides an alternative to aqueous sol–gel formations. The Biosil process is considered as a simple, biocompatible and versatile method for cell immobilization to form functional bio-hybrid materials.34
In view of the above concerns, the aims of the present study were (i) to prepare an immobilized bacterial agent obtained by immobilization of cells on sawdust and coating with silica using the Biosil technology, (ii) to evaluate the viability of immobilized cells using both the usual plate-counting technique and the LIVE/DEAD® BacLight™ kit combined with flow cytometry to discriminate the live, injured and dead cells, and (iii) to investigate the metabolic activity of this immobilized bacteria (Sphingomonas sp. GY2B) toward phenanthrene (Phe). The degradation kinetics of Phe in liquid medium was observed to discuss the removal mechanism of Phe by the immobilized cells. The survival and growth of the inoculated cells and the adsorption–biodegradation of Phe in soil were also studied.
2. Materials and methods
2.1 Materials, organism and soil
Phe (Sigma-Aldrich, purity ≥ 98%) was employed as a model PAH for its prevailing presence in contaminated environments. A stock solution of Phe was prepared by dissolving Phe in n-hexane (10 g L−1). Tetramethyl orthosilicate (TMOS) and HPLC-grade methanol obtained from Shanghai Anpel Scientific Instrument Co., Ltd. All other reagents of analytical grade obtained from Enterprise Group Chemical Reagent Beijing Co., LTD and were used as instructed unless otherwise stated.The carrier used to immobilize bacteria was sawdust selected as the model scaffold. The sawdust was obtained from a nearby furniture processing shop. After being sieved through 20–40 mesh and washed with tap water, the sawdust was dried at 105 °C for 12 h. Before serving as an immobilization host for bacteria, the sawdust was sterilized at 121 °C for 30 minutes, and the process was repeated thrice.
Phe-degrading bacterial strain Sphingomonas sp. GY2B used in the study was previously isolated by our research group. The composition of mineral salt medium (MSM) used for incubation of GY2B was modified from Tao et al.35
Soil samples were taken from a riverside park of the Pearl River, at a depth ranging from 5 to 20 cm. After being air dried and sieved through 2 mm mesh, the soil shows the following physicochemical characteristics: a texture containing sandy loam with pH 6.5, organic matter 0.555%, total nitrogen 0.017%, total phosphorus 0.024%, available nitrogen 31 mg kg−1 and available phosphorus 3 mg kg−1.
2.2 Preparation of the immobilized cells
Sphingomonas sp. GY2B was cultured at 30 °C for 24 h (at late-log phase) in MSM with 100 mg L−1 Phe. Then, the above culture (4%, v/v) was transferred to fresh MSM with 100 mg L−1 Phe and 25 g L−1 sawdust and co-cultured at 30 °C for 36 h. After draining off the supernatant with a piece of nylon mesh, the remaining sawdust with bacteria was washed thrice using phosphate buffered saline (PBS, 100 mL PBS composed of 19 mL 0.2 mol L−1 NaH2PO4 and 81 mL 0.2 mol L−1 Na2HPO4·12H2O, pH 7.4). In this way, bacterial cells were simply immobilized on sawdust, namely the physisorbed immobilized cells (Phy-IC).The silica-immobilization cells (Silica-IC) were conducted by TMOS vapor deposition according to the Biosil process.11,12 Briefly, the above Phy-IC were thinly tiled on a glass Petri dish (90 × 15 mm) which was modified with a central glass well (70 mm diameter) for holding the Phy-IC. TMOS (1 mL) was injected into the outer ring of glass dish, which was filled with glass beads to increase the surface area for evaporation of TMOS (Fig. 1). Then, the glass dish was covered, sealed, incubated for 2 h at 30 °C and stored at 4 °C before use.
Morphological analysis of the silica-coated sawdust was performed by scanning electron microscopy (SEM, ZEISS Evo 18). Energy dispersive spectroscopy (EDS) was also conducted during SEM analysis to confirm the particulate structure. Prior to observation, the samples were freeze dried and then coated with a thin layer of gold.
2.3 Holding capacity of the immobilized matrix
Holding capacity of carriers for the immobilized cells was investigated by a simple flow-through experiment.11 Briefly, approximately 0.5 g sample of Phy-IC or Silica-IC (dry weight) was securely placed on the bottom of a 30 mL plastic syringe. Then the sterilized PBS (250 mL) was pumped through the Phy-IC or Silica-IC at a flow rate of 10 mL min−1 using an up-flow method. Before and after elution, number of cells on sawdust was determined by plate-counting method, as described below.2.4 Long-term storage stability of the immobilized bacteria
Both Phy-IC and Silica-IC were stored at 4 °C and 30 °C for 28 days in order to evaluate the immobilized cell viability and storage stability. The colony forming units (cfu) at the initial time and 28 days later were determined by plate-counting method.36 Briefly, approximately 0.2 g sample of Phy-IC or Silica-IC (wet weight) was re-suspended in 1 mL sterilized normal saline (0.85% NaCl) and disrupted by vortex for 30 minutes. Then, the supernatant was serially diluted with normal saline and counted by agar plates (10 g L−1 peptone, 5 g L−1 beef extract, 5 g L−1 NaCl and 2% agar). After being dried at 105 °C for 12 h, net weights of the left carriers in tubes were determined with electronic balance.2.5 Flow cytometry (FCM) analysis
An alternative viability assay was carried out using the SYTO9/PI staining agent (LIVE/DEAD® BacLight™ bacterial viability kit, Invitrogen, CA).37 For free bacterial cells (FC), after being cultured in MSM for 36 h, cells of GY2B were collected by centrifugation at 6000 rpm for 10 minutes, washed twice and re-suspended in PBS. The cell density was adjusted to approximately 106 cfu mL−1 before staining test. For the immobilized cells, approximately 0.2 g sample of Phy-IC or Silica-IC was re-suspended in 1 mL PBS and then disrupted by vortex for 30 minutes. After standing for a while, the supernatant was harvested, and the method for obtaining the cells was same with the above-mentioned FC.The prepared cell suspension (1 mL) was stained with the SYTO9/PI solution (3 μL, ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature for 20 minutes in the dark, and then sieved through a 400-mesh nylon screen before analysis on a flow cytometer (EPICS XL-MCL, Beckman Coulter, USA). An argon laser at 15 mW (λ488 nm) was utilized as excitation. The emission detectors used for the cells stained with SYTO9 (green fluorescence) and PI (red fluorescence) were 530/30 nm band-pass filter (FL1) and 670 nm long-pass filter (FL3), respectively. The data were acquired until approximately 10
1) at room temperature for 20 minutes in the dark, and then sieved through a 400-mesh nylon screen before analysis on a flow cytometer (EPICS XL-MCL, Beckman Coulter, USA). An argon laser at 15 mW (λ488 nm) was utilized as excitation. The emission detectors used for the cells stained with SYTO9 (green fluorescence) and PI (red fluorescence) were 530/30 nm band-pass filter (FL1) and 670 nm long-pass filter (FL3), respectively. The data were acquired until approximately 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 events were completed. Finally, analysis of data was done using FlowJo software.
000 events were completed. Finally, analysis of data was done using FlowJo software.
In addition, the unstained cells were used as the negative control. PI-positive control (cells with hyperthermia inactivation) and SYTO9-positive control (cells at log-phase) were employed to determine areas of intact, injured and dead cells.
2.6 Phe degradation experiment in liquid
2.7 Phe degradation experiment in soil
In general, 10 mL of dichloromethane were added to 2 g of soil or 0.2 g of sawdust that had been placed in a 30 mL glass centrifuge tube. The sample was extracted using ultra-sonication at 300 watts for 1 hour and then centrifuged at 4000 rpm for 30 minutes. Subsequently, 3 mL of supernatant was filtered through a 2 g silica gel column using 12 mL eluant of hexane and dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to a heart-shaped flask. Finally, the solvent fraction was rotary-evaporated, and then the left in the flask was dissolved with methanol. After being filtered through a 0.22 μm membrane, the sample was analyzed by HPLC.
1, v/v) to a heart-shaped flask. Finally, the solvent fraction was rotary-evaporated, and then the left in the flask was dissolved with methanol. After being filtered through a 0.22 μm membrane, the sample was analyzed by HPLC.
2.8 Analysis of the data
All experiments were carried out in triplicate. The data were analyzed by one-way analysis of variance (ANOVA) using Origin Pro 8.0 software.3. Results and discussion
3.1 Characterization and viability of Silica-IC
In this case, TMOS in vapor phase undergoes rapid hydrolysis upon contact with the water layer present on the surface of material. Further condensation and cross-linking of the hydrolyzed silica monomers occur and lead to formation of the sol–gel and particulate silica.12,33 As a result, direct contact between cells and silica precursors is avoided, so that co-solvents or catalysts which are commonly used in conventional sol–gel synthesis are eliminated, and toxic methanol/alcohol byproducts are immediately evaporated.30 Therefore, the vapor deposition is simple and versatile enough for combination with the abundant natural materials.
The effective immobilization of cells is likely due to the combination of hydrogen bonding and electrostatic interactions between silica particles and material surfaces.33,34 Essentially, the matrix of carriers coated with a silica layer is a bio-inspired approach, which is inspired by single cell microalgae (sponges and diatoms) living inside a porous silica shell ‘frustule’39 and mimics the behavior of bacteria in a natural biofilm (the exo-polysaccharide ‘glue’ that binds cells).11 Therefore, this method of cell immobilization significantly eliminates the biofilm formation time and provides a homogenous population of bacteria with a high and predefined cell density.12
Noticeably, the plate-counting technique, which gives the number of culturable bacteria that remain able to form colonies in the presence of a culture medium, is just one of the methods available for assessing the cell viability.41 As a response to a stress situation, bacterial cells usually enter the viable but non-culturable (VBNC) state.36 In this case, the cell viability may not be reliably assessed by the plate count technique after extraction from carriers. Therefore, an alternate viability assay was tested by SYTO9/PI staining agent combined with FCM to monitor cell membrane integrity and then discriminate the live, injured and dead cells.
FCM based on light-scattering and fluorescent signals is a powerful cell analysis technique at single cell level by multiple parametric analysis of cells.42 During FCM analysis, the forward scattering (FSC) is usually associated with cell sizes, while the side scattering (SSC) is closely related to intracellular particles of cells. The principle of live/dead staining agent in FCM is based on the difference in cell membrane penetration by the nucleic acid fluorescent dyes SYTO9 (green fluorescence) and PI (red fluorescence).37,43 SYTO9 permeates both live and dead bacteria due to its high permeability, while PI only penetrates dead cells where the membrane is severely damaged. Therefore, the fractions of cells in different physiological conditions can be differentiated.
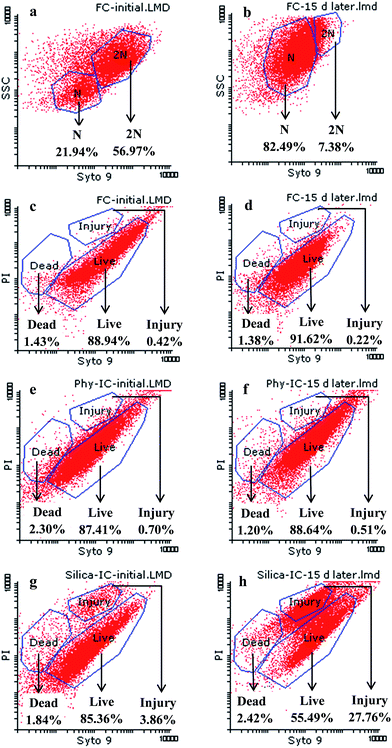
In this study, the changes of DNA ploid for the bacterial source (free cells) were analyzed before and after storing at 4 °C for 15 days. Meanwhile, proportions of live, injured and dead cells for the free cells and immobilized cells were present in Fig. 3. As shown in Fig. 3a, the cells of bacterial source which were harvested at stationary phase mainly contained the DNA diploid (2N). After being stored at 4 °C for 15 days (Fig. 3b), the DNA diploid significantly declined, that meant proportions of cells undergoing division declined in the process of storage. Meanwhile, as shown in Fig. 3c and d, live cells maintained a high proportion (above 88.9%) and had no obvious changes. Live cells in Phy-IC also kept a high proportion (Fig. 3e and f). However, the percentage of live cells in Silica-IC was down to 30% (Fig. 3g and h), and the proportion of injured cells was increased 23.9%, suggesting that cell membranes were partially injured during storage. Even so, the silica coating by vapor deposition did not much damage the integrity of cell membranes, as shown in Fig. 3e and g, the ratios of dead cells in Phy-IC and Silica-IC were below 2.5%.
Combined Fig. 2 with Fig. 3, it was concluded that cells in Silica-IC were able to survive for long periods of time at 4 °C, with maintaining the culturability at least 30 days, while the membrane permeability of Silica-IC was increased, i.e., the proportion of live cells declined and the proportion of injured cells increased. Our previous studies44 demonstrated that the enhanced membrane permeability to a certain degree promoted the bacterial metabolic activity. The injured cells still maintained some enzymatic activity, behaving just as a bag of enzymes.39,41 Nassif et al.45 also mentioned that the bioactivity of immobilized cells, following the usual Michaelis–Menten law, was better than that of free cells. One possible reason was that the lysis of cell membranes resulting from immobilization made substance diffusion easier and then accelerated kinetics of enzymatic reaction. Therefore, the metabolic activity of Silica-IC toward Phe was investigated by the following liquid and soil experiments.
3.2 Phe degradation process in liquid medium
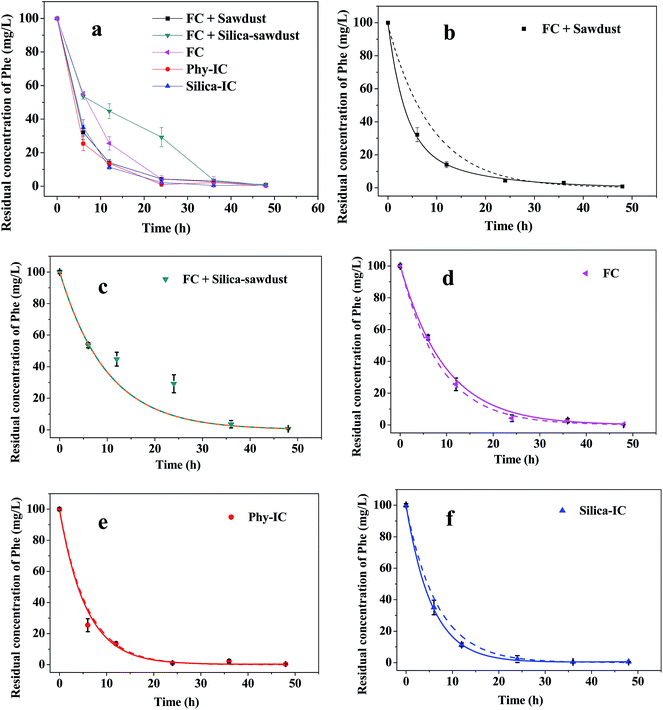
Kinetics of Phe degradation in liquid medium was studied to explore the degradation mechanism of Phe by the immobilized cells. It was found that the immobilized cells were efficient to remove Phe. During 6–36 h, compared to FC, the removal of Phe by Phy-IC and Silica-IC was obviously accelerated (Fig. 4a). Moreover, the Phe removal by free cells was improved by sawdust yet was inhibited by silica-sawdust. Additionally, the adsorption percentage of Phe by sawdust was 38.6% and that by silica-sawdust was 41.6% (after 72 h, data not shown), suggesting that the silica coating on the surface of sawdust did not inhibit Phe adsorbing on the carriers, i.e., Phe could diffuse through the silica coating.The kinetics curves, shown in Fig. 4, are described and interpolated according to a first-order kinetics model and a double-exponential model:46
C = C0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−kt) exp(−kt)
| (1) |
C = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−λ1t) + B exp(−λ1t) + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−λ2t) exp(−λ2t)
| (2) |
Simulated results (Fig. 4b–f and Table 2) show that the Phe removal process by FC is more suitable to the first-order kinetics model, while that by Phy-IC, Silica-IC and FC + Sawdust are more suitable to the double-exponential model which is based on a sorption–degradation process hypothesis. However, neither model is appropriate for the treatment of FC + Silica-sawdust.
| Treatments | The double-exponential model C = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−λ1t) + B exp(−λ1t) + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−λ2t) exp(−λ2t) |
The 1st kinetics model C = C0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−kt) exp(−kt) |
More suitable model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | λ1 | λ2 | R2 | C0 | k | R2 | ||
| FC + Sawdust | 73.1110 | 26.8994 | 0.2933 | 0.0673 | 0.9941 | 98.6109 | 0.11116 | 0.9807 | Double-exponential |
| FC + Silica-sawdust | 95.8648 | 0 | 0.0674 | 0 | 0.9016 | 95.7466 | 0.0677 | 0.9508 | Neither |
| FC | 100.5885 | 0 | 0.1054 | 0 | 0 | 100.9649 | 0.11753 | 0.9984 | The 1st kinetics model |
| Phy-IC | 99.8651 | 0.0676 | 0.1768 | −0.0345 | 0.9974 | 99.8161 | 0.16885 | 0.9934 | Double-exponential |
| Silica-IC | 99.9578 | 0.0496 | 0.1807 | −0.0514 | 0.9999 | 99.3646 | 0.14816 | 0.9791 | Double-exponential |
Based on the above results, we know that a fraction of immobilized cells in liquid are able to be released from the carriers (Table 1), and the carriers are able to adsorb parts of Phe. Therefore, the Phe degradation process by GY2B strain in liquid can be briefly described in Fig. 5. In FC, cells directly use Phe without much limit (Fig. 5a), and according to the first-order kinetics model, the half-life of Phe is 5.9 h. When free cells and sawdust without cells were separately added, i.e., in FC + Sawdust, the sawdust played a role in adsorbing both Phe and cells, resulting in Phe degradation being efficient (Fig. 4a) and fitting a sorption–degradation model (Table 2). A possible reason is that the degradation process occurs not only in the liquid medium but also in the sawdust matrix (Fig. 5b), leading to accelerate the degradation. However, in FC + Silica-sawdust, the degradation was inhibited by the silica coating (Fig. 4a), and neither model mentioned in this study was appropriate. It is speculated that the nano-scale silica coating permits the true solution Phe pass through it and be adsorbed onto sawdust, yet keeps out parts of the micron-size of bacterial cells (Fig. 5d). When bacterial cells were beforehand immobilized below the silica coating in Silica-IC, the degradation effectiveness was improved and high (Fig. 4a), fitting the sorption–degradation model (Table 2). It indicates that the immobilization carrier plays a very important role in the degradation process, and the silica coating does not restrain the transfer and diffusion of Phe (Fig. 5e), compared to Phy-IC (Fig. 5c).
Pannier et al.40 confirmed that the silica-gel coating was mechanically and chemically stable. Compared to cells immobilized in the biocer or alginate beads, bacterial cells, which were immobilized by impregnation of clay pellets in a traditional aqueous nano-sol containing bacteria, were efficient to remove fuel-oxygenates and had the longest storage stability. Luckarift et al.11 also reported that the highly effective degradation might be attributed to that (i) the immobilization method created a high and stable bacterial population and (ii) the carriers collected substrates and sequestered substrates near the immobilized bacteria, allowing degradation to take place in situ. The matrix provided a coupled system of adsorption and biodegradation, and the continuous degradation enhanced the adsorption, then the portion of adsorbed substrates was continuously degraded.
3.3 Bacterial survival and Phe degradation in soil
Ultimate purpose of immobilization was to maintain the viability and metabolic activity of inoculated bacteria for bio-augmentation of contaminated environments. As far as we know, no one has studied the effects of bacteria immobilized by coating with a silica layer on sawdust on the bio-augmentation of PAHs contaminated soil. Therefore, the following soil experiments were carried out to investigate the survival and Phe removal effectiveness of the inoculated immobilized cells. | ||
| Fig. 6 Residual Phe concentrations in soil and carriers. (a) Residual Phe in soil (mg kg−1), (b) enlarged view of (a) from day 2, and (c) adsorbed Phe by carriers (mg kg−1 dry soil or 50 g sawdust). | ||
In the soil matrix, Phe was beforehand spiked in soil, while cells were immobilized on carriers. Results from Fig. 6a and b showed that Phe in soil was rapidly removed after co-culturing with the inoculants. These interesting results motivated us to investigate the possibility of Phe adsorption by carriers. Indeed, parts of Phe in soil were adsorbed onto the carriers, especially at the initial time (Fig. 6c). After co-culturing for 6 h, Phe residual concentrations in soil for treatments Phy-IC and Silica-IC were 400 and 391.6 mg kg−1 dry soil, respectively (Fig. 6a), meantime, Phe adsorbed onto sawdust and silica-sawdust were correspondingly 19.8 and 16.3 mg kg−1 dry soil (the amount of release from per kg dry soil containing approximately 50 g sawdust). When Phe concentrations in soil for treatments Phy-IC and Silica-IC were rapidly reduced to 37.3 and 32.8 mg kg−1 dry soil on day 2, Phe adsorbed onto sawdust and silica-sawdust were 2.6 and 1.9 mg kg−1 dry soil, respectively. Therefore, the carriers used for immobilization were able to adsorb Phe from soil, and the adsorption amount of Phe by carriers was related to the concentrations of Phe in soil.
In summary, compared to free cells, immobilized cells showed a survival benefit, presumably due to the favorable environments for bacterial cells provided by the carrier matrix. The carrier matrix as a bulking agent accelerated water, oxygen and nutrients mass transfer and effectively improved the water holding capacity of soil, that was highly relevant to the bacterial growth and PAHs biodegradation.47 Furthermore, the counting of cfu in Silica-IC was always a bit less than that in Phy-IC. One possible reason was that the silica coating made cells more difficult to wash out from sawdust during plate count, compared to sawdust uncoated with silica. This was consistent with the above results as stated in Table 1. On the other hand, it was observed that the method of silica-immobilization allowed cell division and growth in soil. However, previous studies reported that cells directly immobilized in the silica bulk gels (inorganic host) could not exhibit cell division.30,39,45 It appeared that cell proliferation was indeed possible within the silica network, when the immobilized matrices were prepared by the form of silica layer18 or by the two-step hybrid approach.28,31
Furthermore, it was found that the growth of inoculated cells was closely related to the residual amount of the substrate Phe in soil (Fig. 6a and 7). After adapted to new environments for a while, the immobilized cells began to quickly use Phe as a substrate to grow. However, the number of cells in FC was no longer increased, on the contrary, began to decline since day 4, though Phe removal rate of FC was unexpectedly fast. One possible explanation was that the inoculated cells, maintained at a level of 106 cfu g−1 dry soil, were sufficient for bio-augmentation of the present soil with sandy loam texture. This could be also seen from the treatment of Soil, in which the attenuation of Phe was slow and presented a type of convex curve after hysteresis for 6 days, while that was concave curve for the bio-augmented treatments (FC, Silica-IC and Phy-IC). It meant that the indigenous floras were slowly activated by watering and agitation and played a certain role in the removal of Phe, even though at a low level of 105 cfu g−1 dry soil (data not shown).
To sum up, PAHs in soil were generally considered as poor bioavailability due to their hydrophobicity and being easily absorbed into organic particles, based on the assumption that PAHs degradation by microorganisms was limited by the dissolved PAHs in water.48 On the other hand, it was inevitable that degradation hotspots immediately formed around carriers, upon introducing the immobilized cells, leaving the remaining soil short of degradation potential.49 However, in this study, the removal of Phe in soil by the immobilized bacteria was efficient. Moreover, the silica coating did not inhibit the metabolic activity and growth of immobilized bacteria in soil. This efficient results might be attributed to that (i) the carriers used to immobilize bacteria acted as an adsorption agent to concentrate Phe,7,50 resulting in shortening the distance between Phe and degraders and overcoming the mass-transfer limitation, (ii) fractions of Phe adsorbed onto carriers were able to be degraded by microorganisms through direct contact with no desorption,6,7,51 (iii) the carriers stimulated biofilm formation and uncultured bacterial activity on the Phe-containing carriers48,52 and then enhanced Phe degradative gene expression,53 and (iv) as mentioned above, the carrier matrix as a bulking agent could improve the mass transfer of water, oxygen, nutrients and so on.47
In addition, several researchers6,50 proposed and tried to verify an adsorption–degradation mechanism. In the present of carriers, mass transfer of PAHs from soil to carriers with degraders was accelerated. With direct biodegradation of the carrier-associated PAHs by immobilized cells, there would be more sites for PAHs adsorption and then successive biodegradation. Moreover, this process dominated the dissipation of PAHs in the soil-slurry systems. Regonne et al.52 also identified that the mineralization of Phe adsorbed on hydrophobic membranes was faster than Phe dispersed into soil, for that some special uncultured soil bacteria perhaps played an essential role in PAH biodegradation in soil. In a word, the immobilized bacteria were able to survive well and efficiently bio-augment the soil spiked with Phe.
4. Conclusions
A bio-hybrid material was prepared by a two-step approach involving pre-immobilization of bacterial cells on sawdust followed by coating a silica layer through vapor deposition (Silica-IC), combining benefits of natural materials and silica gel. The silica gel layer reduced cell leakage from sawdust and fulfilled its expected role as a strengthening and stabilizing agent. Silica-IC could maintain long-term stability without losing the reproductive capacity. Even if in soil, Silica-IC survived and grew well. Although the proportion of live cells was declined and that of injured cells was increased during storage at 4 °C for 15 days, the metabolic activity of Silica-IC toward Phe was enhanced both in liquid medium and soil. The potential mechanisms were that (i) the increased membrane permeability made substance diffusion easier, and (ii) the process of Silica-IC toward Phe was dominated by an adsorption and direct degradation mechanism, reducing distances between Phe and degraders and further overcoming the mass-transfer limitation. Overall, bacterial cells immobilized in this biocompatible hybrid matrix maintained high viability and metabolic activity, opening the route to develop a wide range of biological materials for its possible applications in bioreactors or bioremediation.Acknowledgements
This study was financially supported by grants of the Guangdong Provincial Science and Technology Project (2014A020217002 and 2016B020242004), the National High Technology Research and Development Program of China (2012AA101403), the Guangdong Natural Science Funds for Distinguished Young Scholar (2015A030306005) and the Tip-top Scientific and Technical Innovative Youth Talents of Guangdong Special Support Program (2015TQ01Z233).References
- L. Y. Wick, N. Pasche, S. M. Bernasconi, O. Pelz and H. Harms, Appl. Environ. Microbiol., 2003, 69, 6133–6142 CrossRef CAS PubMed.
- J. A. Van Veen, L. S. Van Overbeek and J. D. van Elsas, Microbiol. Mol. Biol. Rev., 1997, 61, 121–135 CAS.
- F. Deng, C. Liao, C. Yang, C. Guo, L. Ma and Z. Dang, RSC Adv., 2016, 6, 20654–20663 RSC.
- M. Cassidy, H. Lee and J. Trevors, J. Ind. Microbiol., 1996, 16, 79–101 CrossRef CAS.
- T. Gentry, C. Rensing and I. Pepper, Crit. Rev. Environ. Sci. Technol., 2004, 34, 447–494 CrossRef CAS.
- B. Chen and J. Ding, J. Hazard. Mater., 2012, 229, 159–169 CrossRef PubMed.
- B. Chen, M. Yuan and L. Qian, J. Soils Sediments, 2012, 12, 1350–1359 CrossRef CAS.
- S. Labana, G. Pandey, D. Paul, N. K. Sharma, A. Basu and R. K. Jain, Environ. Sci. Technol., 2005, 39, 3330–3337 CrossRef CAS PubMed.
- S. Laocharoen, P. Plangklang and A. Reungsang, Environ. Technol., 2013, 34, 2587–2597 CrossRef CAS PubMed.
- Z. Bayat, M. Hassanshahian and S. Cappello, Open Microbiol. J., 2015, 9, 48 CAS.
- H. R. Luckarift, S. R. Sizemore, K. E. Farrington, P. A. Fulmer, J. C. Biffinger, L. J. Nadeau and G. R. Johnson, Biotechnol. Prog., 2011, 27, 1580–1587 CrossRef CAS PubMed.
- H. R. Luckarift, S. R. Sizemore, J. Roy, C. Lau, G. Gupta, P. Atanassov and G. R. Johnson, Chem. Commun., 2010, 46, 6048–6050 RSC.
- T. Coradin, N. Nassif and J. Livage, Appl. Microbiol. Biotechnol., 2003, 61, 429–434 CrossRef CAS PubMed.
- D. Avnir, T. Coradin, O. Lev and J. Livage, J. Mater. Chem., 2006, 16, 1013–1030 RSC.
- A. Pannier, M. Mkandawire, U. Soltmann, W. Pompe and H. Böttcher, Appl. Microbiol. Biotechnol., 2012, 93, 1755–1767 CrossRef CAS PubMed.
- G. Carturan, R. Campostrini, S. Diré, V. Scardi and E. De Alteriis, J. Mol. Catal., 1989, 57, L13–L16 CrossRef CAS.
- R. Ciriminna, A. Fidalgo, V. Pandarus, F. Béland, L. M. Ilharco and M. Pagliaro, Chem. Rev., 2013, 113, 6592–6620 CrossRef CAS PubMed.
- C. Depagne, S. Masse, T. Link and T. Coradin, J. Mater. Chem., 2012, 22, 12457–12460 RSC.
- A. Çabuk, T. Akar, S. Tunali and Ö. Tabak, J. Hazard. Mater., 2006, 136, 317–323 CrossRef PubMed.
- T. Akar, Z. Kaynak, S. Ulusoy, D. Yuvaci, G. Ozsari and S. T. Akar, J. Hazard. Mater., 2009, 163, 1134–1141 CrossRef CAS PubMed.
- G. J. Copello, M. P. Pesenti, M. Raineri, A. M. Mebert, L. L. Piehl, E. R. de Celis and L. E. Diaz, Colloids Surf., B, 2013, 102, 218–226 CrossRef CAS PubMed.
- S. Ramachandran, T. Coradin, P. K. Jain and S. K. Verma, Silicon, 2009, 1, 215–223 CrossRef CAS.
- G. S. Alvarez, M. L. Foglia, D. E. Camporotondi, M. V. Tuttolomondo, M. F. Desimone and L. E. Diaz, J. Mater. Chem., 2011, 21, 6359–6364 RSC.
- K. R. Duarte, C. Justino, T. Panteleitchouk, A. Zrineh, A. C. Freitas, A. C. Duarte and T. A. P. Rocha-Santos, Int. J. Environ. Sci. Technol., 2014, 11, 589–596 CrossRef CAS.
- M. Carabajal, M. Perullini, M. Jobbágy, R. Ullrich, M. Hofrichter and L. Levin, Clean: Soil, Air, Water, 2016, 44, 180–188 CrossRef CAS.
- G. J. Copello, A. M. Mebert, M. Raineri, M. P. Pesenti and L. E. Diaz, J. Hazard. Mater., 2011, 186, 932–939 CrossRef CAS PubMed.
- M. V. Tuttolomondo, G. S. Alvarez, M. F. Desimone and L. E. Diaz, J. Environ. Chem. Eng., 2014, 2, 131–136 CrossRef CAS.
- M. Perullini, M. Jobbágy, N. Mouso, F. Forchiassin and S. A. Bilmes, J. Mater. Chem., 2010, 20, 6479–6483 RSC.
- D. J. Dickson, M. D. Luterra and R. L. Ely, Appl. Microbiol. Biotechnol., 2012, 96, 183–196 CrossRef CAS PubMed.
- M. Blondeau and T. Coradin, J. Mater. Chem., 2012, 22, 22335–22343 RSC.
- M. Perullini, M. M. Rivero, M. Jobbágy, A. Mentaberry and S. A. Bilmes, J. Biotechnol., 2007, 127, 542–548 CrossRef CAS PubMed.
- C. Depagne, C. Roux and T. Coradin, Anal. Bioanal. Chem., 2011, 400, 965–976 CrossRef CAS PubMed.
- G. Gupta, S. B. Rathod, K. W. Staggs, L. K. Ista, K. Abbou Oucherif, P. B. Atanassov, M. S. Tartis, G. A. Montano and G. P. López, Langmuir, 2009, 25, 13322–13327 CrossRef CAS PubMed.
- G. Carturan, R. Dal Toso, S. Boninsegna and R. Dal Monte, J. Mater. Chem., 2004, 14, 2087–2098 RSC.
- X. Q. Tao, G. N. Lu, Z. Dang, C. Yang and X. Y. Yi, Process Biochem., 2007, 42, 401–408 CrossRef CAS.
- M. Blondeau, R. Brayner, F. Guyot and T. Coradin, Anal. Methods, 2014, 6, 2429–2431 RSC.
- T. Soejima, K.-i. Iida, T. Qin, H. Taniai and S.-i. Yoshida, FEMS Microbiol. Lett., 2009, 294, 74–81 CrossRef CAS PubMed.
- K. Vanbroekhoven, A. Ryngaert, L. Bastiaens, P. Wattiau, M. Vancanneyt, J. Swings, R. De Mot and D. Springael, Environ. Microbiol., 2004, 6, 1123–1136 CrossRef CAS PubMed.
- N. Nassif and J. Livage, Chem. Soc. Rev., 2011, 40, 849–859 RSC.
- A. Pannier, C. Oehm, A. R. Fischer, P. Werner, U. Soltmann and H. Böttcher, Enzyme Microb. Technol., 2010, 47, 291–296 CrossRef CAS.
- N. Nassif, C. Roux, T. Coradin, M.-N. Rager, O. M. Bouvet and J. Livage, J. Mater. Chem., 2003, 13, 203–208 RSC.
- M. Díaz, M. Herrero, L. A. García and C. Quirós, Biochem. Eng. J., 2010, 48, 385–407 CrossRef.
- S. Liu, C. Guo, X. Liang, F. Wu and Z. Dang, Ecotoxicol. Environ. Saf., 2016, 129, 210–218 CrossRef CAS PubMed.
- M. L. Zhang, Z. Dang, F. J. Wu, X. J. Liang, C. L. Guo, G. N. Lu and C. Yang, Environ. Sci., 2014, 35, 1449–1456 CAS.
- N. Nassif, O. Bouvet, M. N. Rager, C. Roux, T. Coradin and J. Livage, Nat. Mater., 2002, 1, 42–44 CrossRef CAS PubMed.
- A. Aronne, F. Sannino, S. R. Bonavolontà, E. Fanelli, A. Mingione, P. Pernice, R. Spaccini and D. Pirozzi, Environ. Sci. Technol., 2012, 46, 1755–1763 CrossRef CAS PubMed.
- Y. Liang, X. Zhang, D. Dai and G. Li, Int. Biodeterior. Biodegrad., 2009, 63, 80–87 CrossRef CAS.
- A. R. Johnsen, L. Y. Wick and H. Harms, Environ. Pollut., 2005, 133, 71–84 CrossRef CAS PubMed.
- M. Owsianiak, A. Dechesne, P. J. Binning, J. C. Chambon, S. R. Sørensen and B. F. Smets, Environ. Sci. Technol., 2010, 44, 7622–7627 CrossRef CAS PubMed.
- Y. Dai, L. Yin and J. Niu, Environ. Sci. Technol., 2011, 45, 10611–10618 CrossRef CAS PubMed.
- A. H. Rhodes, M. J. Riding, L. E. McAllister, K. Lee and K. T. Semple, Environ. Sci. Technol., 2012, 46, 12445–12451 CrossRef CAS PubMed.
- R. K. Regonne, F. Martin, A. Mbawala, M. B. Ngassoum and Y. Jouanneau, Environ. Pollut., 2013, 180, 145–151 CrossRef CAS PubMed.
- L. Liu, P. Chen, M. Sun, G. Shen and G. Shang, J. Soils Sediments, 2015, 15, 313–322 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |