Synthesis and hydrophobic properties of F & Si containing poly(ethylene terephthalate)
Hui Shia,
Anbin Tang*ab,
Qianqian Liangb and
Yong Jiangb
aSchool of Material Science and Engineering, Southwest University of Science and Technology, Mianyang 621000, China. E-mail: 1017048558@qq.com
bNational Insulating Material Engineering Research Center, Sichuan EM Technology Co., Ltd, Mianyang 621000, China
First published on 2nd November 2016
Abstract
In this report, a series of F & Si containing poly(ethyl terephthalate) (PET–F–Sis) were synthesized via polycondensation of terephthalic acid, ethanediol, 2,2,3,3,4,4,5,5-octafluoro-1,6-hexanediol and reactive polysiloxane. Reactants had active reactivity and low thermal degradation, and PET–F–Sis had good melting flow property with the η ranging 0.6–0.7 dL g−1. In addition, the production efficiency was increased considerably by decreasing the polycondensation time from 90 minutes to lower than 30 minutes. Moreover, the air–film interfaces of PET–F–Si films were analyzed by water contact angle meter (WCA), X-ray photoelectron spectroscopic (XPS) and atomic force microscopy (AFM). The results indicated that segments containing fluorine and silicon migrated to the surface and fabricated the rough morphology with tiny sharp bulges. And this contributed to the substantial improvement of hydrophobic property of the surfaces with the evidence of the static water contact angle increasing from 76.56° for PET to 134.77° for PET–F–Si10. The progress revealed potential productions of hydrophobic PET materials in future applications.
Introduction
As we know, hydrophobic properties have been highly demanded in industry applications. Hydrophobic surfaces play an important role in extensive application fields, covering the areas of fabric, film and engineering plastic. One of the largest used materials in these regards is poly(ethylene terephthalate) (PET), which possesses a high melting temperature, good chemical properties, good thermal stability, relatively low raw material cost and cost-effective products. In addition to the wide applications in polymeric packages, PET also plays an important role in flexible displays and films of solar cells. Even though many hydrophobic materials have been fabricated, they can only be applied in some special areas. For the common use of hydrophobic materials in large areas, research on hydrophobic PET materials would be very meaningful. However, native PET materials are hydrophilic.1 Improving the hydrophobic property of PET material surfaces can benefit us a lot from daily life to scientific applications. For example, if hydrophobic PET films are used as the cover films of yogurt packages instead of native PET films, we would never need to lick the residual yogurt on the films in order not to waste anymore. Moreover, we can also apply hydrophobic PET materials to make protective textiles, membrane of windows, powder coatings, and so on. But the study on hydrophobic PET is rarely reported so far. To obtain the hydrophobic PET materials, we studied the successful performance from nature to research.Are the interesting water droplets on a lotus leaf when you have been playing outside after it has been raining still fresh in your memory? Besides the lotus leaf, quantities of organisms in nature have been studied for the unique property for water, such as petals,2,3 water strider,4 dragonfly,5 butterfly wing,6 and so on. The phenomena dues to the unique hydrophobic surfaces, which have attracted more and more attentions of researchers, since the control of hydrophobicity on the surface of solid counts a great deal in practical applications.4,7–11 A number of hydrophobic materials have been made and reported to perform well in antibacterial,12 ice-phobic,13 frost-resisting,14 self-cleaning,15 fluid drag reduction,16 etc. Therefore, many efforts have been made to investigate the mysterious of the natural hydrophobic property. As an essential aspect, the unique surface essentially associate to the surface roughness and low surface energy.2,8,11 Through the reported researches, strategies have been carried out on the basis of bio-inspirations, which can be summarized as two methods. One is to create roughness on low surface energy materials. Various methods have been used to fabricate rough surfaces as highly ordered comb-like,17,18 mesoporous,19 multi-scale structures,20 nano-cones,21 and so on. For example, Q. T. Fu developed a ice-phobic coating based on the method of sol–gel.22 H. Zhang fabricated superhydrophobic surface with drag reduction in the way of chemical etching and anodization.23 M. Ramiasa-MacGregor obtained hydrophobic surface by procedure of controlling the size of gold nanoparticles.24 Another method is to add low surface energy component to rough surface materials. Many low surface energy materials especially fluorine and/or silicon-containing materials have been used to obtain hydrophobic surfaces with various rough structures.25,26 X. Chen synthesized fluorinated polyacrylate and fabricated a hydrophobic coating covered with spherical-like particles.27 J. Marczak employed alkylsilanes to the modification of hydrophobic surfaces with roughness in nanoscale.28 H. Maciejewski introduced fluorofunctional organosilicon to obtained superhydrophobic surfaces in cage dimensions of roughness.29 Furthermore, the progress make it possible for us to take advantage of the hydrophobic surfaces from phenomena to research and finally to application.
From now on, the hydrophobicity of PET materials are almost achieved through physical modification with other hydrophobic materials.30,31 In our daily life, hydrophobic raincoat and outdoor tent based on PET materials are most coated with other hydrophobic coatings. Some researchers prepared superhydrophobic PET fabric by coating the PET fabric with SiO2 nanoparticles accompanied with commercial water-repellent agent32 or coating the PET fabric with Al2O3–SiO2 hybrid.33 For the use of solar cell, PET plays as an very important role in the back sheet film, but it's hydrophobicity are gained by polyvinylidene fluoride film. However, they would meet the problems of chipping or peeling off, and would shorten the service life of coatings and films. In addition, we may eat the coatings of food package if it is made in this way. Researchers also used ECR generated sulfur hexafluoride plasma to treat PET substrates and obtained a high hydrophilic/hydrophobic contrast surface.34 Another method is fabricating highly ordered rough surfaces via traditional methods, and we can get different micro/nano morphologies as we expect. Whereas it needs specific equipment, costly and not easy to operate especially for PET materials. What's more, it would also greatly weaken mechanical properties by manufacturing rough structure. Therefore, a promising approach for hydrophobic PET materials is to introduce low surface energy component into PET chains. By adjusting the technology to obtain the proper parameter, the modified PET can be directly made into durable hydrophobic products reducing or cancelling post treatment process. At the same time, terminal waste can be recycled for polyester synthesis, and the technology is real green for environmental protection. Considering the high reaction temperature and harsh reaction pressure of PET, it is important to chose appropriate modification reactants, which is the main problem we need to solve.
In this article, we designed a simple and cost-effective plan to fabricate hydrophobic PET via copolymerization mainly by –OH and –COOH. In consideration of the high cost of fluorine monomer, we used reactive polysiloxane together with only a small amount of 2,2,3,3,4,4,5,5-octafluoro-1,6-hexanediol. At first, chemical structure of the copolymers were characterized by nuclear magnetic resonance (1H NMR and 13C NMR). Then, the activity of produce and process was evaluated by intrinsic viscosity (η), carboxyl end group value ([–COOH]) and polycondensation time (tp). Finally, static water contact angles (WCA), X-ray photoelectron spectroscopy (XPS) and atomic force microscope (AFM) were applied to investigate surface properties of these films. It demonstrated that PET copolymers containing F & Si were made, and the hydrophobic properties of films were greatly improved, which may contribute to the produce of hydrophobic PET materials with potential applications in scientific and practical researches.
Experimental
Materials
Terephthalic acid (TPA) and ethylene glycol (EG) were supplied by Yangtze Petrochemical Co., Ltd. And they were both in industrial purities used for produce PET materials in Sichuan EM Technology Co., Ltd. 2,2,3,3,4,4,5,5-Octafluoro-1,6-hexanediol (OFHD) was purchased from Shanghai Wurui Chemical Co., Ltd. The purity of OFHD was 98%. Reactive polysiloxane (RPSi) was obtained from Shin-Etsu Chemical Co., Ltd. The viscosity of RPSi is 130 mm2 s−1 (25 °C) and it contains 35 g mol−1 functional group equivalent of –OH. The other chemical reagents including antimony trioxide, triphenyl phosphate, lithium acetate, deuterated trifluoroacetic acid, CHCl3, phenol, ortho-dichlorobenzene (ODCB), KOH, and absolute ethanol were all commercially available and used as received without further purification.Synthesis of F & Si containing poly(ethylene terephthalate)s (PET–F–Sis)
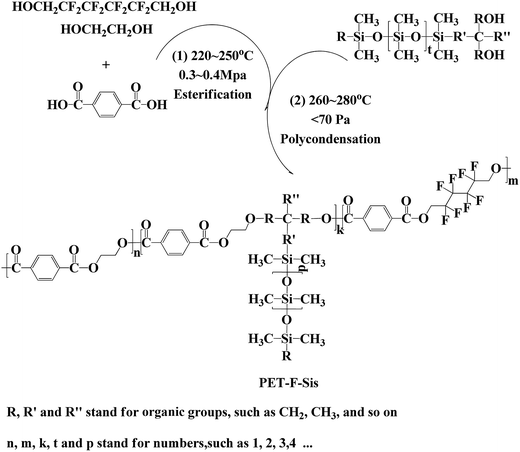
The main synthesis procedure of F & Si containing poly(ethylene terephthalate) (PET–F–Sis) was illustrated in Scheme 1. The synthesis was carried out in a 2 L polyreactor equipped with both esterification system and polycondensation system. In the beginning, feed in PTA and EG (mole ratio 1/1.5) mixed with OFHD (1% mass ratio of PTA). After poured with nitrogen, the reaction system was kept at 220–250 °C and 0.3–0.4 MPa for about 1 h. When the esterification reaction was finished along with the water being removed 90% of the theoretical value, lower the temperature to 200 °C. Subsequently, add antimony trioxide, triphenyl phosphate, lithium acetate and RPSi (0, 2%, 4%, 6%, 8%, 10% mass ratios of the PTA). Heat the system up to 260–280 °C, and built it into high vacuum (<70 Pa) at the beginning of the polycondensation. Stir until the stirring power reach the specified value (50 W/40 Hz). Then end the reaction by filling N2 into the system to atmospheric pressure. The different F & Si containing copolymers were discharged with nitrogen boosting and named as PET, PET–F–Si2, PET–F–Si4, PET–F–Si6, PET–F–Si8, PET–F–Si10. For further study, the PET–F–Sis were dissolved in a solution of CHCl3/phenol (mass ratio 3/2), stirring at 90 °C for 3 h. Absolute ethanol was added dropwise into the solution. The white precipitate was separated by vacuum filtration and was dissolved and precipitate for three times. After being dried at 100 °C in vacuum for 24 h, purified PET–F–Sis were obtained. And were used for characterization of 1H NMR spectra (1H NMR (CF3COOH): δ (ppm) = 8.26, 8.23, 7.95, 7.84, 7.58, 7.50, 7.35, 4.95, 4.82, 4.33, 4.24, 3.16, 0.62, 0.58, 0.56, 0.53, 0.49, 0.36, 0.34, 0.33, 0.30, 0.27 ppm.) and 13C NMR spectra (13C NMR (CF3COOH): δ (ppm) = 171.83, 165.36, 164.93, 164.50, 164.06, 136.57, 133.09, 121.80, 118.98, 116.17, 113.35, 72.01, 67.56, 67.19, 38.12, 19.80, 0.38 ppm).Preparation of PET–F–Si films
The procedure of fabricating films were carried out as the method reported.25,26,29 1 g purified PET–F–Sis were dissolved in 10 g solution of CHCl3/phenol (mass ratio 3/2), being stirred for 3 h at 90 °C. Add the above solution dropwise onto clean glass substrates by using inject needles. The PET–F–Si films were formed at 70 °C in oven for 3 h and 100 °C in vacuum for 12 h. Then cooled to room temperature very slowly and soaked in water for seconds, the PET–F–Si films were stripped from glasses.Characterizations
1H NMR and 13C NMR spectra of PET–F–Sis were obtained on a Bruker AV 600 spectrometer (400 MHz) at 25 °C. Trifluoroacetic acid was used as the solvent and tetramethyl silane (TMS) was used as the internal standard. The intrinsic viscosity (η) of polymers were performed on Ubbelohde viscometer at 25 ± 0.01 °C. ODCB/phenol (volume ratio 1/1) was used as the solvent. Carboxyl end group value ([–COOH]) was calculated through the acid–alkali neutralization titration twice and calculate the average value. At first, dissolve 1 ± 0.005 g the PET–F–Sis in 50 mL solution of CHCl3/phenol (mass ratio 1/1). After that, titration was carried out at room temperature with 2 drops indicator of bromophenol blue, being dropped by the standard solution of KOH–ethanol (0.05 g mL−1) until pure blue. Polycondensation time (tp) was recorded from the beginning of the polycondensation to the end of the reaction. The static water contact angles (WCA) of the PET–F–Si films were performed on German Kruss DSA30 research contact angle measuring instrument at room temperature with the sessile drop method (2 μL droplets). The surface fluorine and silicon content of PET–F–Si films was conducted by X-ray photoelectron spectroscopy (XPS), which was measured by PHI QUANTERA-II SXM energy spectrometer with a Mono Al Kα X-ray source. Japan 300H SPA atomic force microscope (AFM) operated in tapping-mode was applied to reveal the surface roughness and images of PET–F–Si films.Results and discussion
Synthesis of PET–F–Sis
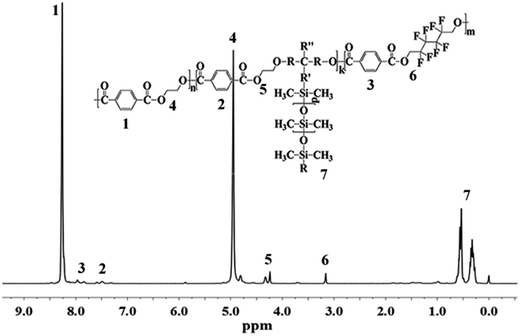
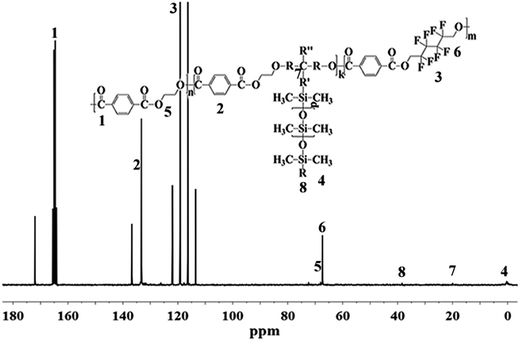
The chemical structure of PET–F–Sis were confirmed by 1H NMR and 13C NMR, which were showed in Fig. 1 and 2. Because the amount of OFHD and RPSi were very little, the characteristic peaks for them were much weaker in the 1H NMR spectrum. In the 1H NMR spectrum, the chemical shifts between 7.35 ppm and 8.26 ppm (peak 1, 2, 3) were due to the phenyl ring in the three constitutional units respectively. The peaks between 4.24 ppm and 4.95 ppm (peak 4, 5) were attributed to the –CH2CH2– group connected to the oxide, whereas the protons on the –CH2– group connected to the –CF2– group appeared at the peaks around 3.16 ppm (peak 6). The existence of multi shifts between 0.27 ppm and 0.62 ppm (peak 7) indicated the characteristic chemical peaks of Si–CH3 group on the RPSi.In the 13C NMR spectrum, the shift at 171.83 ppm was assigned to the peak of trifluoroacetic acid. The chemical shifts between 164.06 ppm and 165.36 ppm (peak 1) were due to the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O connected with phenyl ring and methylene. The signal at 133.09 ppm and 136.57 ppm (peak 2) were attributed to the phenyl ring. The structure of perfluorinated hexamethylene were confirmed by the four shifts between 113.75 ppm and 119.38 ppm (peak 3). The shift at 67.56 ppm (peak 5) was due to the –CH2CH2–, and the shift at 67.19 ppm (peak 6) was due to the –CH2– connected with oxide and –CF2–. The other carbon peaks at 38.12 ppm (peak 8), 19.80 ppm (peak 7) and 0.38 ppm (peak 4) were the characteristic chemical shifts of RPSi. Therefore, the results demonstrated the successful synthesis of the target copolymers with OFHD and RPSi.
C–O connected with phenyl ring and methylene. The signal at 133.09 ppm and 136.57 ppm (peak 2) were attributed to the phenyl ring. The structure of perfluorinated hexamethylene were confirmed by the four shifts between 113.75 ppm and 119.38 ppm (peak 3). The shift at 67.56 ppm (peak 5) was due to the –CH2CH2–, and the shift at 67.19 ppm (peak 6) was due to the –CH2– connected with oxide and –CF2–. The other carbon peaks at 38.12 ppm (peak 8), 19.80 ppm (peak 7) and 0.38 ppm (peak 4) were the characteristic chemical shifts of RPSi. Therefore, the results demonstrated the successful synthesis of the target copolymers with OFHD and RPSi.
Considerable results of the intrinsic viscosity (η), carboxyl end group value ([–COOH]) and polycondensation time (tp) were showed in Table 1. η is the measure of the fluid viscosity for melted PET and the characterization of molecular weight of PET. Normally the η of fiber-grade PET is 0.645 dL g−1. Since the synthesis of PET is based on the reaction of –OH and –COOH, the [–COOH] of PET was the value of residual functional group on the ends of molecular chain which was not react with –OH or emerged in heat degradation. So we can estimate the degree of reaction by the value of carboxyl end group in PET. Thus, η and [–COOH] were very important quality indexes in producing and processing PET materials, because they indicated the molecular mass distribution, the reactivity and thermal degradation indirectly. In production, we need to manage the reaction conditions in order to discharge the melt reaction product easily and avoid degradation. Besides keeping the temperature higher than the melting point of PET (about 250 °C) and lower than 290 °C, it is important for us to control the value of η in appropriate stage.
| Copolymers | η (dL g−1) | [–COOH] (mol t−1) | tp (min) |
|---|---|---|---|
| PET | 0.682 | 18.3 | 90 |
| PET–F–Si2 | 0.669 | 18.7 | 30 |
| PET–F–Si4 | 0.674 | 17.2 | 25 |
| PET–F–Si6 | 0.652 | 18.7 | 22 |
| PET–F–Si8 | 0.665 | 19.0 | 22 |
| PET–F–Si10 | 0.691 | 18.5 | 25 |
As we can see from the results, η of polymers changed in a small range of 0.6–0.7 dL g−1. It indicated that they were in proper polymer molecular weight and had good melt flow property, which were easy for us to discharge the melt reaction product meanwhile conducive to process. In Scheme 1, we can find out that in the main materials of reaction PTA is the only material functionated with –COOH. Then it is easy for us to judge the reaction degree of functional group by measuring the [–COOH]. It showed that the [–COOH] fluctuated diminutively in 17–19 mol t−1, while the normal [–COOH] value of PET is in a wide range of (18–36) ± 4 mol t−1. Therefore it implied that functional groups (–OH and –COOH) of reactants had active reactivity and low thermal degradation, and had a high reaction degree. At last, the production efficiency was estimated by polycondensation time (tp). Reaching the same stirring power of 50 W/40 Hz, tp decreased a lot from 90 minutes to lower than 30 minutes. It indicated that the throughput of PET materials was greatly increased in this polycondensation modification. Therefore, it was a satisfy progress for the production of hydrophobic PET materials.
Surface properties of PET–F–Si films
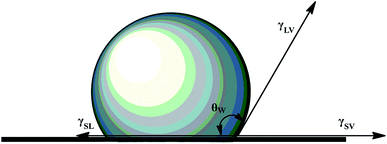
The static water contact angle was used as the criterion for the evaluation of the surface hydrophobic property. For solid–liquid–vapor interfaces of these PET–F–Si films, static water contact angle (θw) was modeled in the famous Young's equation in terms of interfacial energy as eqn (1).4,7,35
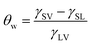 | (1) |
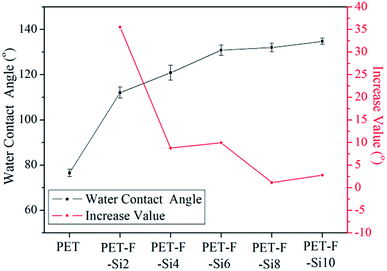
In eqn (1), γSV, γSL and γLV were used to signify the interfacial energy of solid–vapor, solid–liquid, liquid–vapor, respectively. At the same time, Young's contact regime of static water contact angle (θw) was measured as Fig. 3. We can classify the surfaces on the basis of θw to be superhydrophilic (θw < 10°), hydrophilic (θw < 90°), hydrophobic (θw > 90°), superhydrophobic (θw > 150°).36,37 The θw and increase value of them for the air–film interfaces of PET–F–Si films are measured in Fig. 4. And the images are exhibited in Fig. 5. It showed that PET film is hydrophilic with low θw (76.56°). However, after modification via copolymerization, PET–F–Si films intrinsically turned to hydrophobic. When OFHD and RPSi are incorporated in the copolymers with only 1 mass% and 2 mass% of PTA, the θw surprisingly increased to 112.10° for PET–F–Si2. It was a significant increase value for about 35°, highlighted the prominent effect of OFHD and RPSi for the hydrophobicity. As we kept OFHD in the same amount (1 mass% of PTA) and increased the amount of RPSi to 4 mass%, 6 mass%, 8 mass%, 10 mass%, the θw increased to 120.89°, 130.86°, 132.01° and 134.77°, respectively. In contrast, the increase value of θw for PET, PET–F–Si2, PET–F–Si4, PET–F–Si6, PET–F–Si8 and PET–F–Si10 in order slowed down from 35° to about 2°. However, it revealed the highest θw of 134.77° for the first time on the air–film interfaces of PET films. The effective increase suggesting that low surface energy components of fluorine and silicon contribute greatly to the hydrophobicity. Moreover, according to the dynamic changing degree of θw, the fluorine content monomer may played a more important role than silicon component for this improvement.38 Previous research also reported that it is easier for low surface energy segments to migrate with the less amount of them.39
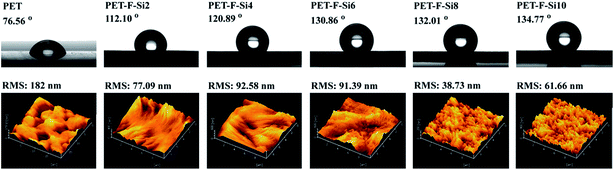 | ||
| Fig. 5 Images of water contact angle and 10 μm × 10 μm 3D morphology for PET–F–Si film surfaces respectively. | ||
XPS analysis of film surfaces was conducted to explore how the fluorine and silicon components contribute to the hydrophobic surfaces. The different elements (C/O/F/Si) content and atomic ratios (F/C and Si/C) of PET–F–Si film surfaces were exhibited in Table 2. The carbon concentration of the surfaces decreased systematically as fluorine and silicon contents increased. This accorded with related researches, which reported that F and Si containing segments tended to aggregate on the air–film interfaces.17,27,40 In addition, the concentration of silicon on the surface increased accordingly to the increase of RPSi. But for PET–F–Si8 and PET–F–Si10, the concentration of silicon on the surface were both around 20%, which did not grow in step with the amount of RPSi. This could be explained as the factor that silicon segment on the surface had reach the saturation value. But there didn't existed enough evidence to support the surmise that the less amount of low surface energy segments the easier for them to migrate to the surface. As we could see, PET film surface without fluorine and silicon was detected to have a θw of about 76.56°. For PET–F–Si2, little amount of F and Si arose on the film surfaces and θw increased substantially to 112.10°. It indicated that F and Si containing segments were effective in improving the hydrophobic performance. Subsequently, increasing only the content of RPSi, F/C changed limitedly ranging from 0.0105 to 0.0164, and Si/C gradually increased from 0.0654 to 0.3992, while θw increase from 120.89° to 134.77°. Therefore, it supported that F containing segments contribute more in hydrophobicity of the air–film interfaces than Si containing segments.
| Samples | Atomic concentration (%) | Atomic ratio | ||||
|---|---|---|---|---|---|---|
| C 1s | O 1s | F 1s | Si 2p | F/C | Si/C | |
| PET | 73.58 | 26.42 | 0 | 0 | 0 | 0 |
| PET–F–Si2 | 65.60 | 29.42 | 0.69 | 4.29 | 0.0105 | 0.0654 |
| PET–F–Si4 | 63.78 | 29.09 | 0.77 | 6.36 | 0.0121 | 0.0997 |
| PET–F–Si6 | 57.80 | 29.50 | 0.94 | 11.76 | 0.0163 | 0.2035 |
| PET–F–Si8 | 55.84 | 24.40 | 0.68 | 19.09 | 0.0122 | 0.3419 |
| PET–F–Si10 | 51.85 | 26.59 | 0.85 | 20.70 | 0.0164 | 0.3992 |
For further investigate, AFM in tapping mode was applied to characterize the 3D image information. The air–film interface morphology for each PET–F–Si films was demonstrated in Fig. 5. In these 3D morphologies, brighter domain and dark domain were contrasted to be different segments of polymers,41 indicating the uneven distribution on the air–film interfaces. PET film surface appeared to have a RMS roughness of 182 nm. Meanwhile, other films with F and Si components copolymerized in had a RMS roughness ranging from 38.73 nm to 92.39 nm, which had decreased obviously. This change was the same as previous report.29 It reveals that the fluorine and silicon contributed to the decrease of RMS on the surfaces of PET films. The value of RMS had decreased around 105 nm from 182 nm for PET to 77.09 nm for PET–F–Si2, and big hollows in the 3D image of PET film didn't showed up in the 3D images of PET–F–Si films. It should caused by the enrichment of fluorine and silicon on the surface. For PET–F–Si films, the RMS were not in a regular figures but in a range from 38.73 nm to 92.39 nm. And we can see that in Fig. 5 the surfaces of PET–F–Si2, PET–F–Si4, PET–F–Si6 were slightly warped, which would also affect the RMS of PET–F–Si films. So the enrichment of fluorine and silicon on the surfaces of films caused orderly arrangement which contributed to the decrease of RMS. While the operation on fabricating the films brings other effects to the RMS of PET–F–Si films, so that the RMS of PET–F–Si films were not in a special trends. However, PET film surface seemed to be more smooth in the brighter morphology, and films contain F and Si seemed to be more rough on which quantities of tiny sharp bulges arose. This phenomenon could be explained by the surface enriching of F and Si segments.24,29,38,42 For PET–F–Si2, there were some tiny sharp bulges attached to structured strips in one orientation. It should be the enrich of F and Si on the surface that fabricate the special structure. Afterwards, along with the increasing amount of polysiloxane, rough morphology became more apparent with intensive sharp bulges, and the orientation became invisible, which should be caused by the concentration of Si on the surface. Thus the fluorine and silicon gathered on the surface of film, which decreased the RMS and created micro or nano structures for the PET–F–Si films.
So that, the hydrophobicity of these films was affected by two factors: low surface energy component (F and Si) and surface roughness/morphology.19,29,36,43 With F and Si integrated, visible morphological changes existed on the air–film interfaces, and the water contact angle began to increase. When F/C and Si/C increased from zero to 0.0105 and 0.0654, one-directional arrangement appeared in the morphology of AFM image and θw increased about 35°. This suggesting that fluorine and silicon containing components created the rough surface and improve the hydrophobicity evidently. After that, increase value of θw was no more than 15°, even though the content of Si on the surface increase substantially and the morphology becomes so hackly. Therefore, Si containing segment could increase the roughness of the surface and also contribute to the increasing of θw. However, by contrasting the increase value of PET–F–Sis with different adding amount of OFHD and RPSi, OFHD provided much higher value in improving the hydrophobicity of the film surface than RPSi.
Conclusions
In present study, fluorine component (2,2,3,3,4,4,5,5-octafluoro-1,6-hexanediol) and silicone component (reactive polysiloxane) were successfully synthesized into poly(ethyl terephthalate) via polycondensation. The copolymers (PET–F–Sis) had good reactivity and low thermal degradation, and the production efficiency was greatly increased. Additionally, they also had a good melting flow property, beneficial for us to produce and process into products of fiber, film and engineering plastics. Effects of fluorine and silicon containing segments on the film surfaces were studied by combining water contact angle meter (WCA), X-ray photoelectron spectroscopic (XPS) and atomic force microscopy (AFM). Consequently, it indicated that the fluorine and silicon components played an important role in the hydrophobicity of the film surface. After chemical connected with fluorine and silicon containing component, the air–film interfaces of PET–F–Sis were covered with tiny sharp bulges, and the water contact angle greatly increased from 76.56° to 134.77°. It is for the first time to obtain a remarkable hydrophobic property on the air–film interfaces of PET films by copolymerization. However, it demand more estimation and improvement to put it into industrial production. Further study will be carried out to investigate the hydrophobic PET materials for promising application.Acknowledgements
The authors acknowledge the support from Scientific Research Fund of Sichuan Provincial Education Department 13TD0022 and Youth innovation team of science and technology of Sichuan Provincial Science and Technology Department 2016TD0014.References
- A. E. Özçam, K. E. Roskov, R. J. Spontak and J. Genzer, J. Mater. Chem., 2012, 22, 5855 RSC.
- L. Feng, Y. Zhang, Y. Cao, X. Ye and L. Jiang, Soft Matter, 2011, 7, 2977 RSC.
- Z. Cheng, M. Du, H. Lai, N. Zhang and K. Sun, Nanoscale, 2013, 5, 2776 RSC.
- S. Yu, Z. Guo and W. Liu, Chem. Commun., 2015, 51, 1775 RSC.
- D. E. Mainwaring, S. H. Nguyen, H. K. Webb, T. Jakubov, M. Tobin, R. Lamb, A. H. Wu, R. Marchant, R. J. Crawford and E. P. Ivanova, Nanoscale, 2016 10.1039/c5nr08542j.
- G. D. Bixler and B. Bhushan, Nanoscale, 2014, 6, 76 RSC.
- P. Ragesh, V. Anand Ganesh, S. V. Naira and A. S. Nair, J. Mater. Chem. A, 2014, 2, 14773 CAS.
- Y.-L. Zhang, H. Xia, E. Kim and H.-B. Sun, Soft Matter, 2012, 8, 11217 RSC.
- Y. Zhang, Y. Chen, L. Shi, J. Li and Z. Guo, J. Mater. Chem., 2012, 22, 799 RSC.
- S. Hu, Z. Xia and L. Dai, Nanoscale, 2013, 5, 475 RSC.
- J. Cai and L. Qi, Mater. Horiz., 2015, 2, 37 RSC.
- X. Zhang, L. Wang and E. Levänen, RSC Adv., 2013, 3, 12003 RSC.
- H. Sojoudi, M. Wang, N. D. Boscher, G. H. McKinley and K. K. Gleasona, Soft Matter, 2015 10.1039/c5sm02295a.
- J. Zhao, A. Meyer, L. Ma and W. Ming, Chem. Commun., 2013, 49, 11764 RSC.
- P. Wang, M. Chen, H. Han, X. Fan, Q. Liu and J. Wang, J. Mater. Chem. A, 2016 10.1039/c6ta01082b.
- G. D. Bixler and B. Bhushan, Nanoscale, 2013, 5, 7685 RSC.
- J. Tan, W. Liu, H. Wang, Y. Sun and S. Wang, Prog. Org. Coat., 2016, 94, 62 CrossRef CAS.
- S. Qin, H. Li, W. Z. Yuan and Y. Zhang, J. Mater. Sci., 2012, 47, 6862 CrossRef CAS.
- H. Yang, W. You, Q. Shen, X. Wang, J. Sheng, D. Cheng, X. Cao and C. Wu, RSC Adv., 2014, 4, 2793 RSC.
- H. Cho, J. Jeong, b. D. C. Wook Kim, S. Lee and W. Hwang, J. Mater. Chem. A, 2016 10.1039/c6ta02159j.
- L. Wang, M. Wen, M. Zhang, L. Jiang and Y. Zheng, J. Mater. Chem. A, 2014, 2, 3312 CAS.
- Q. T. Fu, E. J. Liu, P. Wilson and Z. Chen, Phys. Chem. Chem. Phys., 2015 10.1039/c5cp03243a.
- H. Zhang, L. Yin, L. Li, S. Shi, Y. Wang and X. Liu, RSC Adv., 2016, 6, 14034 RSC.
- M. Ramiasa-MacGregor, A. Mierczynska, R. Sedev and K. Vasilev, Nanoscale, 2016, 8, 35 RSC.
- Y. Xue, H.-C. Lu, Q.-L. Zhao, J. Huang, S.-G. Xu, S.-K. Cao and Z. Ma, Polym. Chem., 2013, 4, 307 RSC.
- S.-S. Zhang, S.-K. Cao, S. Wang, Q.-L. Zhao, J.-Z. Chen, K. Cui and Z. Ma, RSC Adv., 2015, 5, 91140 RSC.
- X. Chen, J. Zhou and J. Ma, RSC Adv., 2015, 5, 97231 RSC.
- J. Marczak, M. Kargol, M. Psarski and G. Celichowski, Appl. Surf. Sci., 2016 DOI:10.1016/j.apsusc.2016.02.071.
- H. Maciejewski, J. Karasiewicz, M. Dutkiewicz, M. Nowicki and Ł. Majchrzycki, RSC Adv., 2014, 4, 52668 RSC.
- M. Seon Han, Y. Park and C. H. Park, Fibers Polym., 2016, 2, 241 Search PubMed.
- T. Y. Kumeeva, N. P. Prorokova and G. A. Kichigina, Prot. Met. Phys. Chem. Surf., 2015, 51, 428 CrossRef.
- G. Y. Bae, Y. G. Jeong and B. G. Min, Fibers Polym., 2010, 11, 976 CrossRef CAS.
- N. P. Damayanti, J. Sol-Gel Sci. Technol., 2010, 56, 47 CrossRef CAS.
- M.-J. Chuang and A.-K. Chu, Appl. Surf. Sci., 2011, 257, 3943 CrossRef CAS.
- T. Qian, J. Wang, T. Cheng, X. Zhan, Q. Zhang and F. Chen, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 2040 CrossRef CAS.
- B. P. Binks and A. T. Tyowua, Soft Matter, 2013, 9, 834 RSC.
- B. N. Sahoo and B. Kandasubramanian, RSC Adv., 2014, 4, 22053 RSC.
- D. Han, L. Zhu, Y. Chen, W. Li and L. Feng, J. Fluorine Chem., 2013, 156, 38 CrossRef CAS.
- D. Han, L. Zhu, Y. Chen, W. Li, X. Wang and L. Ning, RSC Adv., 2015, 5, 22847 RSC.
- T. Xu, H. Liu, J. Song, S.-b. Shang, Z. Song, K. Zou and C. Yang, J. Appl. Polym. Sci., 2015, 132, 41888 Search PubMed.
- D. Han, L. Zhu, Y. Chen, W. Li, X. Wang and L. Ning, J. Appl. Polym. Sci., 2015, 132, 41926 Search PubMed.
- I. Savva, M. Demetriou, A. Othonos, R. Turcu, A. Popa, S. Macavei and T. Krasia-Christoforou, RSC Adv., 2012, 2, 8741 RSC.
- T. Darmanin and F. Guittard, Soft Matter, 2013, 9, 5982 RSC.
| This journal is © The Royal Society of Chemistry 2016 |