Preparation of polyacrylamide microspheres with core–shell structure via surface-initiated atom transfer radical polymerization
Abstract
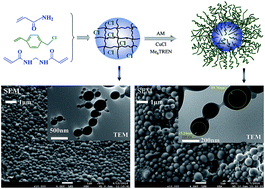
Well defined core–shell microspheres were prepared by surface-initiated atom transfer radical polymerization with pre-crosslinked polyacrylamide as the core and non-crosslinked polyacrylamide as the shell. The obtained microspheres have diameters less than 1 μm with a few tens of nanometers shell thickness. The polymerization kinetics, number-average molecular weight and dispersity were investigated. The materials were characterized with infrared spectroscopy (IR), nuclear magnetic resonance (1H-NMR) spectroscopy, thermogravimetric analysis (TGA), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The viscosity and swelling property of materials were also investigated. The incremental oil recovery obtained with these microspheres is higher than that with polyacrylamide under the same conditions.

 Please wait while we load your content...
Please wait while we load your content...