Ultrathin-nanosheet-assembled Bi2MoO6 mesoporous hollow framework for realizing optimized sunlight-driven photocatalytic water oxidation†
Yuchen Hao,
Xiaoli Dong*,
Xiuying Wang,
Hongchao Ma and
Xiufang Zhang
School of Light Industry and Chemical Engineering, Dalian Polytechnic University, #1 Qinggongyuan, Dalian 116034, PR China. E-mail: dongxl@dlpu.edu.cn
First published on 18th October 2016
Abstract
Herein, a Bi2MoO6 mesoporous hollow framework composed of atomically-thin nanosheets was fabricated for the first time using a one-step solvothermal route. The novel mesoporous hollow structure and ultrathin subunits simultaneously endow the as-prepared catalyst with robust light harvesting ability, excellent charge separation efficiency and abundant surface active sites, leading to improved photocatalytic performance for water oxidation.
Faced with a global energy and resource crisis, semiconductor photocatalysis, which can achieve solar-to-chemical energy conversion effectively, holds great promise for developing an efficient pathway for converting and storing renewable energy.1 Among various photocatalysts, orthorhombic Bi2MoO6, which is constructed from alternating fluorine-like layers (Bi2O2)2+ and perovskite layers (MoO4)2−, possesses high chemical stability, appropriate band-gap energies and suitable valance band potential, and thus exhibits great promise for photocatalytic water oxidation.2 However, the conventional Bi2MoO6 catalyst usually suffers from poor water oxidation efficiency, which is mainly ascribed to its inefficient light-harvesting, serious carrier recombination and very low number of exposed surface active sites. Therefore, an effective optimization strategy for overcoming all the above bottlenecks and further enhancing the water oxidation activity is quite necessary.
Currently, ultrathin two-dimensional (2D) oxide-based semiconductors are regarded as an excellent platform for promoting photocatalytic water oxidation by endowing the catalyst with high light-absorption ability, abundant surface active sites and increased two-dimensional conductivity.3 In particular, the ultra-small longitudinal dimensions can facilitate the fast transfer of excitons from the interior to the catalyst surface, and can thus suppress the bulk charge recombination effectively.4 Moreover, the ultra-large specific surface area, which can efficiently promote sufficient contact between the catalyst and reactants/incident light, is very beneficial for promoting the exposure of surface active sites and solar energy harvesting.5 However, unfortunately, the significant blue-shift of the optical window due to the quantum size effect (QSE) seriously limits the photocatalytic activity of 2D ultrathin photocatalysts.6 On the other hand, hierarchical three-dimensional (3D) hollow materials with a mesoporous structure have also been widely designed for application in light-driven water oxidation.7 The complex pore structure and large specific surface area are very beneficial for promoting multiple light absorption, the diffusion of active species and, especially, the broadening of the optical window as compared to the 2D ultrathin structure. Accordingly, it is of great interest to fabricate photocatalytic active materials with hierarchical 3D mesoporous hollow structures that consist of 2D ultrathin nanosheets, which make them promising as photocatalysts for efficient photocatalytic water oxidation.
Here, inspired by the aforementioned insight, hierarchical Bi2MoO6 mesoporous hollow spheres, constructed from 2D ultrathin nanosheets (UA-Bi2MoO6), were synthesized for the first time via a dual-induced solvothermal driven strategy (Fig. 1). During the solvothermal reactions, two types of assembly processes occurred simultaneously, which were solvent-induced Ostwald ripening and oleate ion-induced lamellar intermediate formation. In particular, dissociated Bi3+ in solution initially interacted with oleate ions to form stable complexes, on which oleate ions are absorbed to reduce the surface energy and promote the formation of an ultrathin structure.6b Meanwhile, the solvent effect from the ethylene glycol/ethanol mixed solution can regulate the self-assembly of the mesoporous hollow structure through a dissolution–recrystallization process (if using water as the solvent, only ultrathin Bi2MoO6 nanosheets can be obtained; Fig. 1).8 As a result, through precisely regulating the crystallisation time (Fig. S1 and S2, ESI†), the novel mesoporous hollow structure and ultrathin subunits were simultaneously realized through this synergistic assembly process. Furthermore, the synthesis of conventional bulk Bi2MoO6 (C-Bi2MoO6; Fig. S4, ESI†) and ultrathin Bi2MoO6 nanosheets (U-Bi2MoO6; Fig. S5, ESI†) for comparison further confirms the importance of these structural features for high-activity photocatalytic water oxidation. Particularly, the novel mesoporous hollow structure and ultrathin subunits simultaneously endow the as-prepared photocatalyst with robust light absorption ability, a broadened optical window, efficient charge separation and transmission, and abundant surface active sites, thereby leading to the unprecedented enhancement of the photocatalytic water oxidation performance.
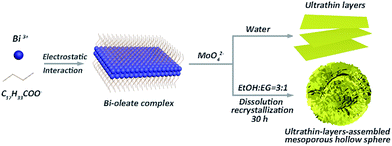 | ||
| Fig. 1 Schematic illustration of the formation of ultrathin Bi2MoO6 layers and ultrathin-nanosheet-assembled Bi2MoO6 mesoporous hollow spheres. | ||
The morphology of the as-prepared sample was investigated using field-emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). The representative FESEM image of the as-prepared UA-Bi2MoO6 catalyst reveals a flower-like spherical morphology. From a broken sphere, the hollow structure of the as-prepared sample was observed (Fig. 2a), which is also verified by the obvious contrast between the dark shell and bright space in the TEM image (Fig. 2b). In addition, the high-resolution SEM (Fig. 2c) and TEM (Fig. 2d and S6, ESI†) images demonstrate that the hollow spheres are assembled from many ultrathin nanosheets with a sub-3.5 nm average thickness, which is approximately two times that of the single unit cell thickness along the (001) direction. Moreover, the marked interplanar spacings of the (200) and (060) planes indicate that the ultrathin subunits exposed (001) facets (Fig. 2e inset). Moreover, the elemental mapping images (Fig. 2f) reveal a uniform distribution of O, Mo and Bi elements throughout the material, suggesting the high purity of the as-prepared sample. In addition, the XRD pattern of as-prepared UA-Bi2MoO6 could be perfectly indexed to orthorhombic Bi2MoO6 (JCPDS no. 84-0787), which fully verifies the pure phase of the as-prepared sample (Fig. S7, ESI†). Meanwhile, the incontestable evidence extracted from the XPS analysis (Fig. S8, ESI†) and FT-IR spectrum (Fig. S9, ESI†) further confirmed the formation of pure Bi2MoO6. Furthermore, the mesoporous structure of UA-Bi2MoO6 was further verified using nitrogen adsorption/desorption isotherms (Fig. S10, ESI†).
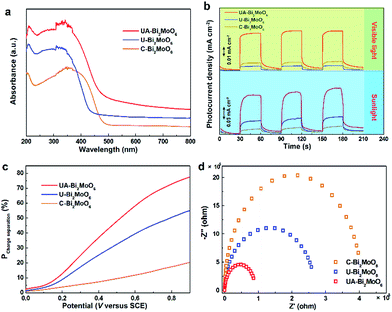
In order to confirm the feasibility of our strategy for optimizing the sunlight-driven photocatalytic water oxidation performance of the Bi2MoO6 catalyst, the changes in the light-harvesting ability, surface active area and charge transfer efficiency with the evolution of the structure from conventional bulk to ultrathin-nanosheet-assembled mesoporous hollow spheres were comprehensively investigated. As indicated by the UV-vis diffuse reflectance spectra (Fig. 3a), the U-Bi2MoO6 catalyst exhibits increased photo-absorption performance in the UV region compared with C-Bi2MoO6, which is mainly due to its large light absorption area. However, owing to the quantum size effect (QSE), the optical window of the U-Bi2MoO6 catalyst exhibits a significant blue-shift, which is unfavorable for the utilization of visible light. Nevertheless, after inducing the assembly of the ultrathin nanosheets into a 3D mesoporous hollow structure, the optical absorption band edge exhibited an obvious red-shift, which can efficiently broaden the photo-absorption region from 450 nm to 520 nm. Furthermore, the novel mesoporous structure can also enhance the multiple scattering of incident light, and thus endows the UA-Bi2MoO6 catalyst with excellent light-absorption ability.9 To further verify the above conjecture, the time-dependent photocurrent measurements of the different samples were performed under simulated sunlight and visible light irradiation and are presented in Fig. 3b. Expectedly, UA-Bi2MoO6 not only exhibits the most remarkable photoelectric conversion performance under simulated sunlight irradiation, but also achieves a significant photocurrent response under visible light irradiation. Such results incontrovertibly indicate that the mesoporous hollow structure and ultrathin subunits lead to a synergistic enhancement in sunlight absorption, which can enhance the light absorption ability and broaden the optical window simultaneously, and thus efficiently promote the efficient energy coupling between photons and excitons.10
To investigate the effects of the structure optimization on charge separation and transmission, the carrier separation efficiency was investigated using a comparison study between the photocurrent density obtained through oxidizing Na2SO3, as well as the theoretical maximum photocurrent density (see the ESI†).11 As presented in Fig. 3c, the ultrathin Bi2MoO6 nanosheet exhibits better exciton separation efficiency than the bulk Bi2MoO6 catalyst, mainly owing to the ultrathin thickness which endows it with an ultrasmall carrier diffusion distance, which can efficiently promote the transfer of photo-excited carriers from the interior to the catalyst surface, thereby leading to an excellent separation efficiency of photogenerated electron–hole pairs.12 Furthermore, after assembling the ultrathin nanosheets into mesoporous hollow spheres, the regular arrangement of the subunits can efficiently avoid the partial reunion of the nanosheets, and hence further enhance the charge separation efficiency. To fully confirm this conclusion, the electrochemical impedance spectra (EIS, Fig. 3d) and PL spectra (Fig. S12, ESI†) were also obtained. Obviously, UA-Bi2MoO6 displays the smallest charge transfer resistance among these three samples, indicating the fastest charge transport kinetics, which can efficiently suppress the recombination of photoexcited carriers.
Consequently, benefiting from efficient light harvesting and fast charge separation, as-prepared UA-Bi2MoO6 exhibits an excellent photoelectric conversion efficiency. As shown in Fig. 4a, although the U-Bi2MoO6 catalyst exhibits an enhanced incident photon-to-current conversion efficiency (IPCE) in the UV region, the poor visible light response seriously limits the efficient utilization of solar energy. But, after assembling the ultrathin layers into a mesoporous hollow structure, the IPCE was significantly enhanced not only in the UV region, but also in the visible region. For example, the IPCE of the UA-Bi2MoO6 photoelectrode is about 7.7% at 400 nm, greater than 5.2% and 1.9% for the U-Bi2MoO6 and C-Bi2MoO6 photoelectrodes. Moreover, the UA-Bi2MoO6 catalyst can also realize an effective photoelectric conversion at wavelengths up to 520 nm, while U-Bi2MoO6 and C-Bi2MoO6 are inactive at this wavelength. Such results further confirm the integrated contribution of the ultrathin subunits and mesoporous hollow structure to the PEC performance. Beyond that, the ultrathin subunits and novel mesoporous structure can also endow the catalyst with an abundance of surface active sites. To better verify this viewpoint, the electrochemically active surface area (ECSA) of the different samples was successfully obtained through measuring the double layer capacitance (Fig. 4b).13 Clearly, the ECSA gradually increases with the structural optimization, which means more catalytically active sites are exposed. In particular, the ultra-large surface area of U-Bi2MoO6 is beneficial for providing more surface active sites for photocatalytic water oxidation. Moreover, after assembling the ultrathin layers into a mesoporous structure, the regular arrangement of the subunits and novel mesoporous structure can further promote the exposure of surface active sites.
Thanks to the robust light harvesting ability, excellent charge separation efficiency and abundant surface active sites, the as-prepared UA-Bi2MoO6 catalyst exhibits excellent photocatalytic water oxidation performance under simulated sunlight irradiation. As presented in Fig. 4c, the O2 evolution rate is up to 184 μmol g−1 h−1, which is 2.1 and 6.6 times greater than that of U-Bi2MoO6 and C-Bi2MoO6, respectively. Such results suggest that the novel mesoporous structure and ultrathin subunits of the as-prepared catalyst promote the efficient enhancement of the sunlight-driven water oxidation efficiency. Meanwhile, the water oxidation activity does not exhibit any significant loss after four cycles of repetition tests, which means that the catalyst possesses excellent catalytic stability.
In summary, ultrathin-nanosheet-assembled Bi2MoO6 mesoporous hollow spheres were synthesized for the first time via a dual-induced solvothermal method, and realized the efficient optimization of the photocatalytic water oxidation performance.
Through investigating the effects of structure evolution on the optical window, exciton diffusion length and surface active area, we confirmed that the novel mesoporous structure and ultrathin subunits can simultaneously endow the as-prepared catalyst with robust light-harvesting ability, excellent charge separation efficiency and abundant surface active sites. More importantly, this work not only provided an effective collaborative optimization strategy for enhancing the photocatalytic activity of Bi2MoO6 based photocatalysts, but also experimentally revealed the role of structure evolution in promoting photocatalytic water oxidation performance.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant no. 21476033) and the Cultivation Program for Excellent Talents of Science and Technology Department of Liaoning Province (no. 201402610).Notes and references
- (a) S. S. K. Ma, T. Hisatomi, K. Maeda, Y. Moriya and K. Domen, J. Am. Chem. Soc., 2012, 134, 19993 CrossRef CAS PubMed; (b) S. E. Guo, Z. P. Deng, M. X. Li, B. J. Jiang, C. G. Tian, Q. J. Pan and H. G. Fu, Angew. Chem., Int. Ed., 2016, 55, 1830 CrossRef CAS PubMed; (c) P. Zhang, T. Wang, X. X. Chang, L. Zhang and J. L. Gong, Angew. Chem., Int. Ed., 2016, 55, 5851 CrossRef CAS PubMed; (d) J. W. Huang, Y. Ding, X. Luo and Y. Y. Feng, J. Catal., 2016, 333, 200 CrossRef CAS; (e) C. Zhou, Y. F. Zhao, L. Shang, R. Shi, L. Z. Wu, C. H. Tung and T. R. Zhang, Chem. Commun., 2016, 52, 8239 RSC.
- (a) B. J. Jin, Z. B. Jiao and Y. P. Bi, J. Mater. Chem. A, 2015, 3, 19702 RSC; (b) Z. Dai, F. Qin, H. P. Zhao, F. Tian, Y. L. Liu and R. Chen, Nanoscale, 2015, 7, 11991 RSC; (c) L. W. Zhang, Y. Man and Y. F. Zhu, ACS Catal., 2011, 1, 841 CrossRef CAS; (d) Y. C. Hao, X. L. Dong, S. R. Zhai, X. Y. Wang, H. C. Ma and X. F. Zhang, Chem. Commun., 2016, 52, 6525 RSC; (e) S. N. Lou, J. Scott, A. Iwase, R. Amal and Y. H. Ng, J. Mater. Chem. A, 2016, 4, 6964 RSC.
- (a) Y. W. Liu, H. Cheng, M. Lyu, S. J. Fan, Q. H. Liu, W. S. Zhang, Y. D. Zhi, C. M. Wang, C. Xiao, S. Q. Wei, B. J. Ye and Y. Xie, J. Am. Chem. Soc., 2014, 136, 15670 CrossRef CAS PubMed; (b) C. L. Tan and H. Zhang, Nat. Commun., 2015, 6, 7873 CrossRef CAS PubMed; (c) Z. B. Jiao, H. C. Yu, X. S. Wang and Y. P. Bi, RSC Adv., 2016, 6, 73136 RSC; (d) Y. F. Sun, S. Gao, F. C. Lei and Y. Xie, Chem. Soc. Rev., 2015, 44, 623 RSC.
- (a) D. H. Deng, K. S. Novoselov, Q. Fu, N. F. Zheng, Z. Q. Tian and X. H. Bao, Nat. Nanotechnol., 2016, 11, 218 CrossRef CAS PubMed; (b) S. Gao, Y. Lin, X. C. Jiao, Y. F. Sun, Q. Q. Luo, W. H. Zhang, D. Q. Li, J. L. Yang and Y. Xie, Nature, 2016, 529, 68 CrossRef CAS PubMed; (c) Z. Xing, X. K. Zeng, A. Deletic, D. McCarthy, G. Wang and X. W. Zhang, Chem. Commun., 2016, 52, 6985 RSC; (d) Y. H. Liu, J. H. Xiong, S. G. Luo, R. W. Liang, N. Qin, S. J. Liang and L. Wu, Chem. Commun., 2015, 51, 15125 RSC.
- Q. Han, B. Wang, J. Gao, Z. H. Cheng, Y. hao, Z. P. Zhang and L. T. Qu, ACS Nano, 2016, 10, 2745 CrossRef CAS PubMed.
- (a) Y. G. Zhou, Y. F. Zhang, M. S. Lin, J. L. Long, Z. Z. Zhang, H. X. Lin, J. S. Wu and X. X. Wang, Nat. Commun., 2015, 6, 8340 CrossRef PubMed; (b) L. Liang, F. C. Lei, S. Gao, Y. F. Sun, X. C. Jiao, J. Wu, S. Qamar and Y. Xie, Angew. Chem., Int. Ed., 2015, 54, 13971 CrossRef CAS PubMed.
- (a) Y. T. Xiao, Y. J. Chen, Y. Xie, G. H. Tian, S. E. Guo, T. R. Han and H. G. Fu, Chem. Commun., 2016, 52, 2521 RSC; (b) Q. F. Wu, S. Y. Bao, B. Z. Tian, Y. F. Xiao and J. L. Zhang, Chem. Commun., 2016, 52, 7478 RSC.
- G. H. Tian, Y. J. Chen, W. Zhou, K. Pan, Y. Z. Dong, C. G. Tian and H. G. Fu, J. Mater. Chem., 2011, 21, 887 RSC.
- (a) M. Weiss, S. Waitz, R. Ellinghaus, T. Weller and R. Marschall, RSC Adv., 2016, 6, 79037 RSC; (b) I. Tamiolakis, I. T. Papadas, K. C. Spyridopoulos and G. S. Armatas, RSC Adv., 2016, 6, 54848 RSC.
- H. X. Li, Z. F. Bian, J. Zhu, D. Q. Zhang, G. S. Li, Y. N. Huo, H. Li and Y. F. Lu, J. Am. Chem. Soc., 2007, 129, 8406 CrossRef CAS PubMed.
- (a) H. Dotan, K. Sivula, M. Grätzel, A. Rothschild and S. C. Warren, Energy Environ. Sci., 2011, 4, 958 RSC; (b) P. Yan, G. Liu, C. Ding, H. Han, J. Shi, Y. Gan and C. Li, ACS Appl. Mater. Interfaces, 2015, 7, 3791 CrossRef CAS PubMed; (c) D. K. Zhong, S. Choi and D. R. Gamelin, J. Am. Chem. Soc., 2011, 133, 18370 CrossRef CAS PubMed.
- J. Li, G. M. Zhan, Y. Yu and L. Z. Zhang, Nat. Commun., 2016, 7, 11480 CrossRef PubMed.
- (a) S. Gao, X. C. Jiao, Z. T. Sun, W. H. Zhang, Y. F. Sun, C. M. Wang, Q. T. Hu, X. L. Zu, F. Zhang, S. Y. Yang, L. Liang, J. Wu and Y. Xie, Angew. Chem., Int. Ed., 2016, 55, 698 CrossRef CAS PubMed; (b) M. Popczyk, A. Serek and A. Budniok, Nanotechnology, 2003, 14, 341 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the synthesis procedures, characterization and photocatalytic water oxidation experiments. SEM images, TEM images, XRD patterns, FT-IR spectra, PL spectrum, N2-adsorption–desorption isotherm, and XPS spectrum. See DOI: 10.1039/c6ra22605a |
| This journal is © The Royal Society of Chemistry 2016 |