Schottky–ohmic converted contact, fast-response, infrared PbTe photodetector with stable photoresponse in air†
Abstract
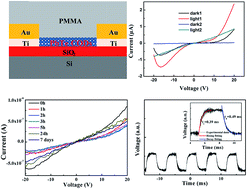
Lead chalcogenide colloidal quantum dots (CQDs) offer promise in infrared photodetectors due to their narrow bandgap and quantum size effect. Compared with PbS and PbSe CQDs, PbTe CQD has received the least attention because it is much easier oxidized in air owing to weak binding energy and hard size control is not in favor of observing quantum confinement effect. Here we developed a layer-by-layer method to prepare PbTe CQD photodetectors with the assistance of the inorganic halide ligand (TBAI) treatment. The results show that TBAI treated PbTe CQD film photodetectors have high photocurrent and short response time (0.39 ms) and recovery time (0.49 ms), indicating the effectiveness of TBAI treatment on PbTe CQD film. We also find PMMA protector is necessary to obtain stable photoresponse performance for TBAI treated PbTe CQD film photodetectors in air.

 Please wait while we load your content...
Please wait while we load your content...