Improvement of selectivity for CO2 reduction by using Cu2ZnSnS4 electrodes modified with different buffer layers (CdS and In2S3) under visible light irradiation†
Sunao
Kamimura
abc,
Yousuke
Sasaki
a,
Masaki
Kanaya
a,
Toshiki
Tsubota
a and
Teruhisa
Ohno
*acd
aDepartment of Applied Chemistry, Faculty of Engineering, Kyushu Institute of Technology, 1-1 Sensuicho, Tobata, Kitakyushu 804-8550, Japan. E-mail: tohno@che.kyutech.ac.jp
bPRESTO, Japan Science and Technology Agency, 4-1-8 Honcho, Kawaguchi-shi, Saitama 322-0012, Japan
cResearch Center for Advanced Eco-fitting Technology, Kyushu Institute of Technology, 1-1 Sensuicho, Tobata, Kitakyushu 804-8550, Japan
dACT-C, Japan Science and Technology Agency, 4-1-8 Honcho, Kawaguchi-shi, Saitama 322-0012, Japan
First published on 24th November 2016
Abstract
Cu2ZnSnS4 (CZTS) electrodes modified with different n-type buffer layers (CdS and In2S3) were used as photocathodes for CO2 reduction under visible light irradiation (420 < λ < 800 nm) in aqueous media. Compared to a bare CZTS electrode, CZTS electrodes modified with n-type buffer layers (CdS and In2S3), by which a p–n heterojunction between CZTS and the n-type buffer layer is formed, showed a significant increase in the photocurrent assigned to CO2 reduction. In addition, product selectivity of CO2 reduction was improved by surface modification with an n-type buffer layer: that is, selective CO2 reduction into CO was achieved by using a CdS/CZTS electrode, while HCOOH selectivity was observed over an In2S3/CZTS electrode. In this study, we investigated the photoelectrochemical properties of CZTS electrodes modified with n-type buffer layers (CdS and In2S3) in conjunction with the structural and optical properties, and we investigated their activity for PEC CO2 reduction.
Introduction
Carbon dioxide (CO2) is the thermodynamically stable product of most fossil fuel combustion processes and is a significant contributor to the greenhouse effect. The accumulation of CO2 in the atmosphere has a great impact on climate change and could threaten the environment and eventually the worldwide economy. The conversion of CO2 into useful products and chemicals is thus a very attractive research area. Photoelectrochemical (PEC) reduction of CO2 to value-added products has been widely studied as a possible technique for the mitigation of atmospheric CO2.1–3 Over the past few decades, tremendous work on PEC CO2 reduction has been done by using semiconductor electrodes in water electrolysis as both an electron donor and a proton source,4,5 a technique is currently called artificial photosynthesis. One way to achieve artificial photosynthesis is to directly convert CO2 into organic fuels over p-type semiconductor electrodes (photocathodes) in combination with n-type semiconductor electrodes (photoanodes) for oxygen generation from water without applying an external voltage under sunlight illumination. There have been many studies on high-efficiency photoanodes such as WO3,6,7 TaON,8,9 Fe2O3,10,11 and BiVO4,12,13 while there have been few reports on photocathodes with a high selectivity for CO2 reduction because a semiconductor electrode generally shows preferential H2 production in an aqueous solution.Cu2ZnSnS4 (CZTS) is one of the promising p-type semiconducting materials for PEC devices due to its ability to absorb visible light (optical band gap of ca. 1.5 eV) and its relative stability in aqueous environments.14–16 In addition, it is made of cheap, non-toxic, earth-abundant elements and can be easily produced on a large scale. PEC CO2 reduction utilizing a CZTS electrode was first reported in 2011 by Arai et al., who carried out a study in which CO2 was reduced to formate with a high selectivity (current efficiency > ca. 80%) by using a Ru complex-modified CZTS electrode under visible light irradiation (400 < λ < 800 nm).17 In the absence of a Ru complex, no formate was produced, indicating that the active center of CO2 reduction might be a Ru complex. The activity for CO2 reduction was also improved by the insertion of Se into the CZTS electrode, presumably due to an improvement of carrier mobility.
We were inspired by their work and became interested in exploring the potential of CZTS electrodes modified with n-type buffer layers (CdS and In2S3) for efficient PEC CO2 reduction reaction. Since the deposition of n-type buffer layers (CdS, In2S3) over the p-type CZTS layer results in the formation of a depletion region at the solid–solid interface (p–n heterojunction), which can also assist in the extraction photo-generated electrons from CZTS toward the n-type buffer layers, the photocurrent is enhanced.18–20 Moreover, colloidal CdS has been studied as a visible light responsive photocatalyst that showed activities for CO2 reduction to form CO with high quantum yields from DMF including sacrificial reagents.21 Li et al. reported that In2S3 exhibited visible-light activity toward photocatalytic reduction of CO2 in the presence of water vapor into renewable CH4.22 Based on these observations, it was expected that enhancement of the photocurrent and improvement of selectivity for CO2 reduction is anticipated by using CZTS electrodes modified with n-type buffer layers (CdS and In2S3). However, as far as we know, there has been no report on PEC CO2 reduction by using n-type buffer layer-modified CZTS electrode. The present study was carried out from this standpoint.
We fabricated a CZTS electrode by the sol–gel spin-coating method on a molybdenum-coated glass substrate. Different n-type buffer layers, such as CdS and In2S3, were deposited on the CZTS electrode by the chemical bath deposition (CBD) method. The bare CZTS electrode exhibited a cathodic photocurrent under visible light irradiation, and the photocurrent reached ca. −100 µA cm−2 at 0 V vs. reversible hydrogen electrode (RHE) in CO2-saturated NaHCO3 solution. The cathodic photocurrent was enhanced by deposition of n-type buffer layers due to efficient charge carrier separation as a result of the creation of a p–n heterojunction, leading to PEC reaction with the primary products being hydrogen, carbon monoxide, and formate. In this study, PEC properties of the CZTS electrode were investigated in conjunction with structural and optical properties, and the contributions of n-type buffer layers (CdS, In2S3) to the CO2 reduction property were investigated.
Experimental
Preparation of Cn2ZnSnS4 electrode
The Cu2ZnSnS4 electrode was fabricated by the sol–gel and spin-coating method using the following procedure.23 First, 0.4 mol L−1 Cu(CH3COO)2·H2O (Wako, 99.0%), 0.25 mol L−1 Zn(CH3COO)2·2H2O (Wako, 99.9%), 0.2 mol L−1 SnCl2·2H2O (Wako, 99.9%) and 1.6 mol L−1 SC(NH2)2 (Wako, 98%) were dissolved in 2-methoxyethanol (10 mL, Wako, 99.0%) containing monoethanolamine (Sigma-Aldrich, >99.0%). The concentration of each metal ion coincides with the generally reported data for high-efficiency solar cells, and an excess amount of thiourea was used to complex metal ions and alleviate the loss of sulfur during the annealing process. To prevent the formation of cracks in the precursor thin film during the sulfurization process, additive monoethanolamine is necessary in the precursor solution. Approximately 70 µL of the precursor solution was dropped on a Mo-coated soda-lime glass (Mo/glass, 18 mm × 20 mm in size, GEOMATEC Co., Ltd.) and then the excess precursor solution was removed by spin-coating (3000 rpm, 30 s). The electrode was dried in air at 200 °C for 5 min on a hotplate. At this time, the precursor solution is considered to be formed metal–thiourea–oxygen complexes, which is stable in air.23 After this process had been repeated for a maximum of 5 times, the electrode was calcined at 560 °C for 1 h in a sulfur/N2 atmosphere. It should be noted that the number of times of spin-coating was optimized in this study (refer Fig. S1 in ESI†). To remove impurity phases such as Cu2−xS over the CZTS film, (NH4)2S etching processes were performed for 6 h. Then a bare CZTS electrode was obtained.Surface modification with n-type buffer layers
The n-type buffer layers (CdS, In2S3) were deposited on the CZTS electrode by the chemical bath deposition (CBD) method using the following procedure. The CZTS electrode was immersed in 0.125 mol L−1 Cd(CH3COO)2·2H2O (Wako, 98.0%), 2.8 mol L−1 SC(NH2)2 (Wako, 98%), and NH3 aqueous solution at 65 °C for 9 min to yield CdS-modified CZTS (CdS/CZTS). For In2S3-modified CZTS (In2S3/CZTS), the CZTS electrode was immersed in an aqueous solution containing 0.025 mol L−1 InCl3·4H2O (Wako, 99.9%), 0.1 mol L−1 CH3CSNH2 (Wako, 98.0%), and 0.1 mol L−1 CH3COOH (Wako, 99.7%) at 65 °C for 15 min. After the CBD techniques, the electrodes were annealed at 200 °C and 100 °C, respectively, and then CdS/CZTS and In2S3/CZTS electrodes were fabricated.Characterization
The crystalline phases were characterized by using a powder X-ray diffraction (XRD) instrument (MiniFlex II, Rigaku Co.) with CuKα (λ = 1.5418 Å) radiation (cathode voltage: 30 kV, current: 15 mA). Raman spectroscopy measurements were performed by using a JASCO NRS 5100 laser Raman spectrophotometer. Absorption properties of the CZTS electrode were determined by using the diffuse reflection method with a UV/VIS/NIR spectrometer (V570, JASCO Co., Japan) attached to an integral sphere at room temperature. Mott–Schottky analysis was carried out by using an electrochemical analyzer (604D, ALS Co.) with a bare CZTS electrode, a platinum electrode, an Ag/AgCl electrode and 0.1 M Na2SO4 + NaOH solution (pH = 9.5) used as a working electrode, counter electrode, reference electrode and electrolyte, respectively. X-ray photoelectron spectroscopy (XPS) measurements were performed by using a Kratos AXIS Nova spectrometer (Shimazu Co.) with a monochromatic Al Kα X-ray source. The binding energy was calibrated by taking the carbon (C) 1s peak of contaminant carbon as a reference at 284.6 eV.Photoelectrochemical (PEC) measurement
PEC performance of the CZTS electrode was investigated in a three-electrode configuration using an Ag/AgCl reference electrode and a Pt coil counter electrode. The electrolyte was CO2-saturated 0.1 M NaHCO3 solution. Linear sweep voltammetry and chronoamperometry measurements were carried out by using an automatic polarization system (HSV-110, Hokuto Denko Co.) under a Xe lamp equipped with an L-42 cut-off filter (SCF-50s-42L, SIGMAKOKI Co., Ltd.) and IR cut-off filter (420 < λ < 800 nm). The scan rate for the linear sweep voltammetry was 10 mV s−1.Analysis of products
PEC CO2 reduction was performed in a gastight three-electrode configuration cell with a double compartment in which a CZTS electrode, Pt coil and silver–silver chloride (Ag/AgCl) electrode were used as a working electrode, counter electrode and reference electrode, respectively. The electrolyte solution was 0.1 M NaHCO3, which was purged with CO2 gas for ca. 40 min prior to the start of measurement. After CO2 bubbling, the cell was sealed and irradiated by visible light irradiation (Xe lamp, 420 nm < λ < 800 nm, 100 mW cm−2) for 1 h. Evolved H2, CO gas was detected by an on-line gas chromatograph (GC) with a thermal conductivity detector (Agilent Technology Co. MicroGC) equipped with an MS-5A column. He gas was used as the carrier gas. The liquid CO2 reduction product, formic acid (HCOOH), was detected by using single-channel ion chromatography (ICS900, Thermo Fisher Scientific Inc.), and other products including methanol (CH3OH), ethanol (C2H5OH), and formaldehyde (HCOH) were detected by gas chromatography (G-3500, Hitachi Co.) with a DB-WAXetr column (122-7332, Agilent Co.).Results and discussion
Structural and optical properties of the CZTS electrode
Fig. 1(a) shows the XRD pattern of the CZTS electrode together with the ICDD standard file of the tetragonal phase of Cu2ZnSnS4 (JCPDS 00-026-0575). An intense peak corresponding to the bottom Mo substrate was observed at 40.02°, while the other diffraction peaks coincided with the reference pattern corresponding to (112), (200), (220), (312), (224) planes of CZTS, indicating that CZTS crystal could be synthesized by the sol–gel and spin-coating method. Fig. 1(b) shows a Raman spectrum of the CZTS electrode under 532 nm laser illumination. There is an intense peak centered at 333 cm−1 along with two weak peaks located at 288 cm−1, and 368 cm−1; these signals are in agreement with those of the kesterite CZTS structure,24–26 and no other peaks were found to be binary phases such as hexagonal Cu2−xS (475 cm−1), Sn2S3 (304 cm−1), ZnS (352 cm−1) and cubic Cu2SnS3 (303 cm−1 and 356 cm−1).27 However, SnO2 phase was observed at 663 cm−1.23 Therefore, our fabricated CZTS electrode consists of the major phase of kesterite CZTS and minor phases of SnO2. Although a slightly weak peak corresponding to hexagonal MoS2 phase was observed at 400 cm−1,23,27 the MoS2 phase plays an important role in the characteristics of the CZTS electrode, as described later. | ||
| Fig. 1 (a) XRD pattern of the CZTS electrode together with that of a reference pattern. (b) Raman spectrum of the CZTS electrode under 532 laser illumination. | ||
Fig. 2 shows top and cross-section SEM photographs of the CZTS electrode. The flat and tight structure of the CZTS film was observed to have good adhesion to the Mo substrate. The thickness of the CZTS layer was estimated to be ca. 400 nm. Moreover, a MoS2 layer was observed at the interface between the CZTS layer and Mo substrate. Formation of the MoS2 layer was inevitable in the sulfurization process of CZTS, which may facilitate electrical quasi-ohmic contact and improve the adhesion of CZTS to the Mo back contact, but leads to high series resistance and accordingly deteriorates the device efficiency if not thin enough. In this study, we optimized the thicknesses of the CZTS layer and MoS2 layer by adjusting the spin-coating and sulfurization processes, respectively.
Fig. 3 shows the diffuse reflectance spectrum of the CZTS electrode. CZTS is a direct energy gap material. The optical absorption of CZTS could be converted to a Tauc plot, (αhv)n vs. hv, where n = 2 for a direct bandgap, by extending the tangential lines to the abscissa. The band gap was estimated to be ca. 1.5 eV, which is well consistent with the reported ones in ref. 18.
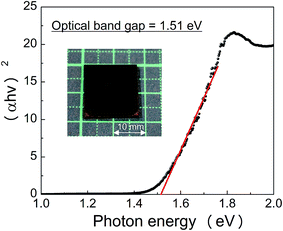 | ||
| Fig. 3 Tauc plot for the CZTS electrode. Inset figure shows a picture of our fabricated CZTS electrode. | ||
Structural and optical properties of buffer layer-modified CZTS electrode
Surface modifications of the CZTS electrode with n-type buffer layers were performed by using the CBD method. Fig. 4 shows top and cross-section SEM photographs for CZTS electrodes modified with n-type buffer layers (CdS and In2S3). Compared to the bare CZTS, the surface morphology was appreciably changed. The surface of the CdS/CZTS electrode was composed of porous grains with sizes on the order of several micrometers. In contrast, the In2S3/CZTS electrode surface was composed of a billowing thin layer. As seen in the cross-section SEM photographs, the thicknesses of CdS and In2S3 buffer layers were estimated to be ca. 50 nm and ca. 60 nm, respectively. For clear confirmation of the formation of In2S3 and CdS layers, the XRD measurement has been done. However, no diffraction peaks of In2S3 and CdS was observed, suggesting that their thicknesses were too thin to be detected by XRD analysis. We therefore carried out the Raman spectroscopy measurement for CdS/CZTS and In2S3/CZTS electrodes together with that for CdS/Mo and In2S3/Mo substrates. As shown in Fig. 5(a), a relatively sharp peak detected from CdS/CZTS/Mo and CdS/Mo substrates at 299 cm−1 was considered to be the longitudinal optical mode Raman reflection of CdS in accordance with literature studies.28,29 This results indicated that the CdS layer could be formed on the CZTS electrode by CBD method. On the other hand, broad peaks were observed at 304 and 368 cm−1 in In2S3/Mo substrate (see Fig. 5(b)). These peaks were attributed to β-In2S3 phase,30,31 implying that crystalline In2S3 could be formed on the CZTS electrode by CBD method. It should be noted that Raman peak of In2S3 was buried under that of CZTS in the case of the In2S3/CZTS/Mo. | ||
| Fig. 4 Top and cross-sectional SEM photographs of (a), (b) the CdS/CZTS electrode and (c), (d) the In2S3/CZTS electrode. | ||
 | ||
| Fig. 5 Raman spectra for (a) CdS/Mo glass and CdS/CZTS/Mo glass and (b) In2S3/Mo glass and In2S3/CZTS/Mo glass. | ||
Fig. 6(a) show the UV-vis spectra of CdS and In2S3 layers which fabricated on the ITO substrate by CBD method. The optical absorption of CdS layer could be converted to a Tauc plot, (αhv)n vs. hv, where n = 2 for a direct bandgap, by extending the tangential lines to the abscissa. On the other hand, a straight plot was obtained for the In2S3 layer by applying n = 1/2 to the Tauc plot due to its indirect bandgap material. From intersects of the linear portions of these (αhv)n vs. hv curves with the photon energy axis (see Fig. 6(b) and (c)), the optical bandgap of CdS and In2S3 buffer layers used in this study were determined to be 2.48 and 2.30 eV, respectively.
Photoelectrochemical property of the CZTS electrode
Fig. 7 shows current–potential curves for bare CZTS, CdS/CZTS and In2S3/CZTS electrodes in 0.1 M NaHCO3 solution purged with CO2 gas with irradiation of visible light (420 < λ < 800 nm). The bare CZTS electrode exhibited a cathodic photocurrent in response to irradiation of incident light, and the photocurrent density reached ca. −160 µA cm−2 at −1.0 V vs. Ag/AgCl. The cathodic photocurrent was increased by ca. 2 times after deposition of n-type buffer layers (CdS, In2S3) because of efficient charge separation in the depletion region of the p–n heterojunction between p-type CZTS and n-type buffer layers. Deposition of Pt contributed to further enhancement of the cathodic photocurrent due to improvement of the surface reaction rate (see Fig. S2 in ESI†); however, the observed photocurrent was attributed to water reduction reaction with the primary product being hydrogen, as will be discussed later. No CO2 reduction product was observed in Pt/CZTS, Pt/CdS/CZTS, and Pt/In2S3/CZTS. Thereafter, we therefore studied PEC CO2 reduction by using bare CZTS, CdS/CZTS and In2S3/CZTS electrodes. | ||
| Fig. 7 Current–potential curves in 0.1 M NaHCO3 solution under chopped visible light irradiation (420 < λ < 800 nm, 100 mW cm−2) for CZTS, CdS/CZTS and In2S3/CZTS electrodes. | ||
Fig. 8 shows time courses of the cathodic photocurrent from bare CZTS, CdS/CZTS and In2S3/CZTS electrodes in 0.1 M NaHCO3 solution purged with CO2 gas at −1.0 V vs. Ag/AgCl under visible light irradiation (420 < λ < 800 nm). For the bare CZTS electrode, the cathodic photocurrent rapidly increased when it was exposed to visible light, and then the photocurrent remained constant with time, implying that the CZTS electrode is stable upon PEC reaction. On the other hand, the cathodic photocurrent for CdS/CZTS and In2S3/CZTS electrodes showed a slight increase up to about 20 min and was relatively stable over the observed time course. A similar tendency was reported by Moriya et al. and by Septina et al., who showed that the CdS buffer layer in Pt/CdS/CuGaSe2 and Pt/CdS/Cu(In,Ga)S2 was dissolved in the electrolyte upon PEC reaction due to photocorrosion of CdS, and the cathodic photocurrent in the electrodes may have increased as the CdS buffer layer became thinner.32,33 Based on above reports, we studied the XPS spectra of bare CZTS, CdS/CZTS and In2S3/CZTS electrodes before and after PEC reaction. For the bare CZTS electrode (see Fig. 9), there was almost no difference in the XPS spectra for Cu 2p, Zn 2p and S 2p before and after PEC reactions, whereas Sn 3d3/2 XPS spectrum was slightly changed: a relatively weak peak was observed at 496.4 eV before PEC reaction, and this peak was attributed to Sn(IV) species in SnO2. After PEC measurement, the SnO2 peak was disappeared, suggesting that a part of the cathodic photocurrent of bare CZTS electrode was consumed in reducing SnO2 impurity phase. For the CdS/CZTS electrode (see Fig. 10(a)), major peaks at 405.1 eV and 411.9 eV were observed before PEC measurement, and these peaks were attributed to typical values of Cd 3d5/2 and 3d3/2, respectively. After PEC reaction, both of peak intensities were decreased, suggesting that the CdS buffer layer became thinner, similar to previous reports described above.32,33 For the In2S3/CZTS electrode (see Fig. 10(b)), major peaks at 444.5 eV and 452.1 eV were observed before PEC measurement, and these peaks were attributed to typical values of In 3d5/2 and 3d3/2, respectively. These peak profile of the In 3d XPS spectrum was clearly changed after PEC reaction; small shoulder peaks located at 443.3 eV and 450.9 eV were newly observed. These peaks were identified to be metal indium In(0). These results indicated that In(III) species in the In2S3/CZTS electrode was gradually reduced to In(0) species by PEC reaction. We speculated that the metal indium In(0) contributed to photocurrent response. That is, metal indium In(0) acts as a co-catalyst of the In2S3/CZTS electrode, which leading to an enhancement of the cathodic photocurrent.
 | ||
| Fig. 9 XPS spectra for Cu 2p, Zn 2p, Sn 3d and S 2p before and after photoelectrochemical measurements of the bare CZTS electrode. | ||
 | ||
| Fig. 10 XPS spectra for Cd 3d and In 3d before and after photoelectrochemical measurements of the CdS/CZTS and In2S3/CZTS electrodes. | ||
Finally, we examined PEC CO2 reduction over bare CZTS, CdS/CZTS and In2S3/CZTS electrodes at −1.0 V Ag/AgCl in CO2-saturated 0.1 M NaHCO3 solution under visible light irradiation (420 < λ < 800 nm, 100 mW cm−2). The results are shown in Table 1, where all of these values are averages of three times (for detail, see the Table S1 in ESI†). For the bare CZTS electrode, H2, CO and HCOOH were detected as products and no formation of CH4, CH3OH and C2H5OH was observed. The thermodynamic potential for PEC reduction of CO2 to CO and HCOOH in the presence of protons is generally explained by the following equation:
| CO2 + 2H+ + 2e− → HCOOH + H2O (−0.61 V vs. NHE) |
| CO2 + 2H+ + 2e− → CO + H2O (−0.53 V vs. NHE) |
| Entry | Sample | CO2 | Coulomb/C | Faradaic efficiency/% | |||
|---|---|---|---|---|---|---|---|
| H2 | CO | HCOOH | Total | ||||
| a Irradiated at visible light (420 < λ < 800 nm, 100 mW cm−2) for 1 h in aqueous 0.1 M NaHCO3 solution at −1.0 V vs. Ag/AgCl. b Under Ar-purged Na2SO4 aqueous solution. | |||||||
| 1 | CZTS | ○ | 0.9 | 74.3 | 1.4 | 2.4 | 78.1 |
| 2 | CZTS | ✗b | 0.5 | 62.7 | 0 | 0 | 62.7 |
| 3 | CdS/CZTS | ○ | 1.5 | 75.0 | 7.4 | 1.1 | 83.5 |
| 4 | In2S3/CZTS | ○ | 1.0 | 72.0 | 3.5 | 4.6 | 80.1 |
| 5 | Pt/CdS/CZTS | ○ | 6.0 | 67.0 | 0 | 0 | 67.0 |
| 6 | Pt/In2S3/CZTS | ○ | 6.0 | 73.4 | 0 | 0.3 | 73.7 |
The conduction potential of the CZTS electrode was estimated to be −1.25 V vs. RHE by Mott–Schottky analysis (see Fig. S3 in ESI†), and the CO2 reduction reactions into CO and HCOOH were therefore thermodynamically favorable. However, the calculated faradic efficiencies for H2, CO and HCOOH were approximately 74.3%, 1.4% and 2.4% (the average of three times), respectively, indicating that most of the cathodic photocurrent of the bare CZTS electrode was derived from H2 evolution. These results indicated that water reduction reaction preferentially occurred over the CZTS electrode in the aqueous solution. In contrast, CO evolution was increased by depositing a CdS buffer layer over the CZTS electrode, and its faradic efficiency increased up to 7%, implying that selectivity for CO2 reduction was improved by using the CdS/CZTS electrode. It should be noted that no CO evolution was observed when PEC measurement was performed in Ar-saturated Na2SO4 aqueous solution (also N2-saturated NaHCO3 aqueous solution) or in a dark condition, suggesting that the evolved CO gas was derived from PEC reduction of purged CO2 gas over the CdS/CZTS electrode. In addition, the Pt-modified CdS/CZTS electrode exhibited H2 evolution with trace CO and trace HCOOH in CO2-saturated NaHCO3 aqueous solution under visible light irradiation. Considering that Pt shows high activity for H2 evolution with a low overpotential, it is highly possible that water reduction reaction preferentially occurred over the Pt/CdS/CZTS electrode rather than CO2 reduction. These results indicate that the CdS buffer layer plays an important role in selective PEC CO2 reduction into CO. Indeed, Yanagida et al. proposed that CO evolution over CdS nanoparticles was caused by adsorption of CO2 molecules at the CdS surface with sulfur vacancies through bidentate-type adsorption of CO2, resulting in selective CO formation.21 Therefore, our observation is reasonable. On the other hand, the In2S3/CZTS electrode generated a relatively large amount of HCOOH compared with that generated by the bare CZTS electrode. Its origin is currently unknown. Since the surface of In2S3/CZTS electrode contained metal indium based on the results of XPS spectra (refer to Fig. 10(b)), it is difficult to identify whether the CO2 reduction reaction occurs at the In2S3 buffer layer or metal indium. Indeed, metal indium is well-known to exhibit a high CO2 selectivity for HCOOH formation in the research field of electrochemistry. In addition, Li et al. reported that In2S3 contributed to visible-light activity toward photocatalytic reduction of CO2 into CH4 without any other products.22 On the basis of those reports, PEC CO2 reduction by using an In2S3/CZTS electrode may occur on the In2S3 surface or metal indium, and thus is likely show selective CO2 reduction into HCOOH. Thus, CZTS electrodes modified with n-type buffer layers (CdS and In2S3) showed a strong photocurrent response and selectivity for CO2 reduction, which was strongly dependent on the kind of buffer layer: that is, CO selectivity was improved by deposition of a CdS buffer layer, while HCOOH selectivity was improved by deposition of an In2S3 buffer layer.
Conclusions
We fabricated a CZTS electrode by the sol–gel and spin-coating method on a Mo/glass substrate and performed photoelectrochemical CO2 reduction under visible light irradiation (λ > 420 nm). The CZTS electrode exhibited a cathodic photocurrent under visible light irradiation; however, H2 evolution (faradaic efficiency > ca. 74%) preferentially occurred over the bare CZTS electrode in the CO2-saturated NaHCO3 solution. By depositing an n-type buffer layer (CdS and In2S3) over the CZTS electrode, enhancement of photocurrent response and improvement of selectivity for CO2 reduction were achieved: that is, CO selectivity was improved by deposition of a CdS buffer layer, and its faradaic efficiency was increased up to ca. 7%. In contrast, HCOOH selectivity was improved by deposition of an In2S3 buffer layer, and its faradaic efficiency was increased up to ca. 5%. The improvement of selectivity for CO2 reduction was related to the kind of n-type buffer layer, and the adsorption of CO2 molecules at the n-type buffer layer plays an important role in selective PEC CO2 reduction. Further research is underway to test this via the deposition of surface co-catalysts that can facilitate PEC CO2 reduction. This study could be applied to improve the selectivity for CO2 reduction by using other Cu–chalcogenide photocathodes.Acknowledgements
The authors thank Prof. Shigeru Ikeda of Konan University and Prof. Tetsuya Haruyama for their valuable contributions to this study. This work was supported by a grant from Advanced Catalytic Transformation program for Carbon utilization (ACT-C), Japan Science and Technology Agency (JST).References
- M. Halmann, Nature, 1978, 275, 115–116 CrossRef CAS.
- M. Schreier, P. Gao, M. T. Mayer, J. Luo, T. Moehl, M. K. Nazeeruddin, S. D. Tilley and M. Grätzel, Energy Environ. Sci., 2015, 8, 855–861 CAS.
- G. Sahara, R. Abe, M. Higashi, T. Morikawa, K. Maeda, Y. Ueda and O. Ishitani, Chem. Commun., 2015, 51, 10722–10725 RSC.
- S. Sato, T. Arai, T. Morikawa, K. Uemura, T. M. Suzuki, H. Tanaka and T. Kajino, J. Am. Chem. Soc., 2011, 133, 15240–15243 CrossRef CAS PubMed.
- T. Arai, S. Sato, T. Kajino and T. Morikawa, Energy Environ. Sci., 2013, 6, 1274–1282 CAS.
- K. Sivula, F. L. Formal and M. Grätzel, Chem. Mater., 2009, 21, 2862–2867 CrossRef CAS.
- J. A. Seabold and K. S. Choi, Chem. Mater., 2011, 23, 1105–1112 CrossRef CAS.
- M. Higashi, K. Domen and R. Abe, Energy Environ. Sci., 2011, 4, 4138–4147 CAS.
- M. Higashi, K. Domen and R. Abe, J. Am. Chem. Soc., 2012, 134, 6968–6971 CrossRef CAS PubMed.
- A. Kay, I. Cesar and M. Grätzel, J. Am. Chem. Soc., 2006, 128, 15714–15721 CrossRef CAS PubMed.
- K. Sivula, R. Zboril, F. Le Formal, R. Robert, A. Weidenkaff, J. Tucek, J. Frydrych and M. Grätzel, J. Am. Chem. Soc., 2010, 132, 7436–7444 CrossRef CAS PubMed.
- K. Sayama, A. Nomura, T. Arai, T. Sugita, R. Abe, M. Yanagida, T. Oi, Y. Iwasaki, Y. Abe and H. Sugihara, J. Phys. Chem. B, 2006, 110, 11352–11360 CrossRef CAS PubMed.
- Y. H. Ng, A. Iwase, A. Kudo and R. Amal, J. Phys. Chem. Lett., 2010, 1, 2607–2612 CrossRef CAS.
- D. Yokoyama, T. Minegishi, K. Jimbo, T. Hisatomi, G. Ma, M. Katayama, J. Kubota, H. Katagiri and K. Domen, Appl. Phys. Express, 2011, 3, 101202 CrossRef.
- L. Rovelli, S. D. Tilley and K. Sivula, ACS Appl. Mater. Interfaces, 2013, 5, 8018–8024 CAS.
- F. Jiang, Gunawan, T. Harada, Y. Kuang, T. Minegishi, K. Domen and S. Ikeda, J. Am. Chem. Soc., 2015, 137, 13691–13697 CrossRef CAS PubMed.
- T. Arai, S. Tajima, S. Sato, K. Uemura, T. Morikawa and T. Kajino, Chem. Commun., 2011, 47, 12664–12666 RSC.
- N. Guijarro, M. S. Prévot and K. Sivula, J. Phys. Chem. Lett., 2014, 5, 3902–3908 CrossRef CAS PubMed.
- P. Wang, T. Minegishi, G. Ma, K. Takanabe, Y. Satou, S. Maekawa, Y. Kobori, J. Kubota and K. Domen, J. Am. Chem. Soc., 2012, 134, 2469–2472 CrossRef CAS PubMed.
- C. Yan, F. Liu, N. Song, B. K. Ng, J. A. Stride, A. Tadich and X. Hao, Appl. Phys. Lett., 2014, 104, 173901 CrossRef.
- H. Fujiwara, H. Hosokawa, K. Murakoshi, Y. Wada, S. Yanagida, T. Okada and H. Kobayashi, J. Phys. Chem. B, 1997, 101, 8270–8278 CrossRef CAS.
- H. Li, Y. Gao, Y. Zhou, F. Fan, Q. Han, Q. Xu, X. Wang, M. Xiao, C. Li and Z. Zou, Nano Lett., 2016, 16, 5547–5552 CrossRef CAS PubMed.
- Z. Su, K. Sun, Z. Han, H. Cui, F. Liu, Y. Lai, J. Li, X. Hao, Y. Liu and M. A. Green, J. Mater. Chem. A, 2014, 2, 500–509 CAS.
- M. Altosaar, J. Raudoja, K. Timmo, M. Danilson, M. Grossberg, J. Krustok and E. Mellikov, Phys. Status Solidi A, 2008, 205, 167–170 CrossRef CAS.
- P. A. Fernandes, P. M. P. Salomé and A. F. da Cunha, Thin Solid Films, 2009, 517, 2519–2523 CrossRef CAS.
- K. Wang, O. Gunawan, T. Todorov, B. Shin, S. J. Chey, N. A. Bojarczuk, D. Mitzi and S. Guha, Appl. Phys. Lett., 2010, 97, 143508 CrossRef.
- P. A. Fernandes, P. M. P. Salomé and A. F. da Cunha, J. Alloys Compd., 2011, 509, 7600–7606 CrossRef CAS.
- O. Zelaya-Angel, F. L. Castillo-Alvarado, J. Avendailo-Lopez, A. Escamilla-Esquivel, G. Contreras-Puente, R. Lozada-Morales and G. Torres-Delgado, Solid State Commun., 1997, 104, 161–166 CrossRef CAS.
- I. O. Oladejia, L. Chowa, J. R. Liub, W. K. Chub, A. N. P. Bustamantec, C. Fredricksena and A. F. Schultea, Thin Solid Films, 2000, 359, 154–159 CrossRef.
- K. Kambas, J. Spyridelis and d. M. Balkan, Phys. Status Solidi B, 1981, 105, 291–296 CrossRef CAS.
- E. Kärber, K. Otto, A. Katerski, A. Mere and M. Krunks, Mater. Sci. Semicond. Process., 2014, 25, 137–142 CrossRef.
- M. Moriya, T. Minegishi, H. Kumagai, M. Katayama, J. Kubota and K. Domen, J. Am. Chem. Soc., 2013, 135, 3733–3735 CrossRef CAS PubMed.
- W. Septina, Gunawan, S. Ikeda, T. Harada, M. Higashi, R. Abe and M. Matsumura, J. Phys. Chem. C, 2015, 119, 8576–8583 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra22546b |
| This journal is © The Royal Society of Chemistry 2016 |



