Up-conversion luminescence behaviors in Er3+ doped single crystal KNbO3 nanosheets
Abstract
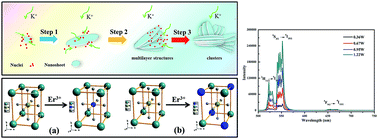
Perovskite oxides have attracted much attention owing to their excellent physical properties, such as piezoelectricity, ferroelectricity, and ferromagnetism. Moreover, these properties can be strongly affected and regulated by the doping strategy. In this work, Er3+ doped single crystal KNbO3 nanosheets were successfully synthesized via a solvothermal method. XRD analysis indicated that Er3+ firstly substituted into the B site (Nb5+) and then A site (K+) in the ABO3 perovskite structure with the further increasing content of Er3+. The orthorhombic structure of the pure KNbO3 and Er3+ doped KNbO3 was proved in terms of the Raman spectra. The SEM observation demonstrated that the size of the as-obtained nanosheets was about 70 nm in thickness and 3 μm in diameter. The single crystal structure state of the as-obtained products was confirmed by HRTEM. Furthermore, the growth mechanism of Er3+ doped KNbO3 nanosheets was rationally elaborated and proposed. The as-obtained Er3+ doped KNbO3 nanosheets exhibited excellent up-conversion (UC) photoluminescence (PL) properties with a strong green emission and a weak red emission. The maximum emission intensity of the UC PL spectrum was achieved with 0.4 mol% of Er3+ doped. To conclude, the as-synthesized Er3+ doped KNbO3 nanosheets could be a potential candidate for the development of multifunctional devices, by introducing PL properties to piezoelectric KNbO3 materials.

 Please wait while we load your content...
Please wait while we load your content...