Real time and in situ observation of graphene growth on liquid metal surfaces via a carbon segregation method using high-temperature confocal laser scanning microscopy†
Abstract
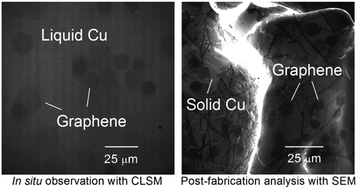
Fabrication processes of single-layer and multilayer graphene (SLG and MLG, respectively) that are simple yet controllable are actively sought-after to realize many graphene-based applications. We report the fabrication, growth kinetics, and characterization of graphene formed on liquid metal surfaces through a segregation method that was determined by combining real time and in situ imaging using a high-temperature confocal laser scanning microscope (CLSM) with high-resolution scanning electron microscopy imaging and Raman spectroscopy. Carbon was first dissolved in liquid copper (silver) at 1300 °C (1200 °C) and then precipitated out as graphene on a liquid copper (silver) surface at 1130 °C or 1090 °C (1000 °C). SLG with a smooth texture formed and almost fully covered the liquid copper surface within seconds but partially covered the liquid silver surface. In contrast to graphene formed on liquid silver, which maintained a smooth texture after solidification of liquid silver and did not possess any MLG, the graphene that formed on liquid copper comprised of SLG and MLG, and developed ripples and wrinkles upon solidification. Real time and in situ observations with a high-temperature CLSM revealed that small MLG precipitates formed between the liquid copper surface and SLG, and were attached underneath the SLG. Interestingly, the precipitates grew into large hexagonal islands instead of coalescing together. Furthermore, occasionally two MLG islands joined into a larger MLG island with individual MLG islands maintaining its hexagonal shape and slowly drifted as a unit on the liquid copper surface, confirming that the MLG islands formed after SLG formation and were attached to the SLG layer. Lastly, the number densities, areal sizes, and thicknesses of these MLG islands strongly depended on graphene formation temperatures and carbon dissolution times. For example, lowering the graphene formation temperature from 1130 °C to 1090 °C with the same graphene formation time as well as the carbon dissolution time and temperature resulted in a greater number of thinner and laterally smaller MLG islands. On the other hand, increasing the carbon dissolution time while keeping the carbon dissolution temperature along with the graphene formation time and temperature the same led to fewer and thicker MLG islands with larger lateral sizes. The real time and in situ observations provide direct insights on graphene formation and growth kinetics, which will allow precise control of graphene quality formed on liquid metal surfaces.


 Please wait while we load your content...
Please wait while we load your content...