Biotransformation of neuro-inflammation inhibitor kellerin using Angelica sinensis (Oliv.) Diels callus†
Di Zhoua,
Ning Li*a,
Yuhua Zhangb,
Chunyan Yanb,
Kun Jiaoc,
Yu Sund,
Hui Nid,
Bin Line and
Yue Hou*c
aSchool of Traditional Chinese Materia Medica 49#, Shenyang Pharmaceutical University, Key Laboratory of Structure-Based Drug Design and Discovery, Ministry of Education, Wenhua Road 103, Shenyang 110016, China. E-mail: liningsypharm@163.com; Fax: +86-24-31509368; Tel: +86-24-23986475
bCollege of Pharmacy, Guangdong Pharmaceutical University, Guangzhou, 510006, PR China
cCollege of Life and Health Sciences, Northeastern University, Wenhua Road 3-11, Shenyang 110004, China. E-mail: houyue@mail.neu.edu.cn
dXinJiang Institute of Chinese Materia Medica and Ethnodrug, Urumqi 830002, China
eSchool of Pharmaceutical Engineering, Shenyang Pharmaceutical University, Shenyang 110016, PR China
First published on 3rd October 2016
Abstract
Kellerin, a sesquiterpene coumarin derivative, has been identified as a major constituent of Ferula sinkiangensis K. M. Shen. It has been proved to be a potential natural therapeutic agent for Alzheimer's disease because of its inhibition of inflammatory cytokines nitric oxide (NO), tumor necrosis factor-α (IL-6) (TNF-α), cyclooxygenase-2 (COX-2), interleukin-6 (IL-6) and interleukin-1β (IL-1β) in over-activated BV2 mouse microglial cells. Because of the multi chiral centers and the chemical instability of the sesquiterpene, coumarin, it is rather difficult to obtain this bioactive natural product by synthesis. Thus, biotransformation of kellerin was carried out to afford more novel derivatives using the callus of Angelica sinensis (Oliv.) Diels, which has an abundance of biosynthetic enzymes of phenylpropanoids. As a result, 14 products were obtained and identified, including four new sesquiterpene coumarin derivatives: 14′-hydroxy-(3′S,4′R,5′S,8′R,9′S,10′R)-kellerin (1), 5′,6′-ene-14′-hydroxy-(3′S,4′R,8′R,9′S,10′R)-kellerin (2), 5′,6′-ene-(3′R,8′R,9′S,10′R)-ferukrin (3), and 14′-hydroxy-(3′S,4′R,5′S,8′R,9′S,10′R)-deacetylkellerin (4), together with 10 other known compounds. Their structures were elucidated using comprehensive spectroscopic techniques and their possible biosynthetic pathways were proposed on the basis of the structural analyses. Furthermore, their anti-neuroinflammatory activities were assessed in BV2 cells by monitoring lipopolysaccharide-induced NO production, and the structure–activity relationships were discussed.
Introduction
Sesquiterpene coumarins are a small family of natural product, which are constructed of a coumarin moiety and a sesquiterpene fragment through an ether linkage. They are usually found in some plants of Umbelliferae, Compositae and Rutaceae families.1 Sesquiterpene coumarins have been reported to be characteristic of the chemical composition of the Ferula plant genus, and are responsible for antiviral, antibacterial, antileishmanial, antitumor and anti-neuroinflammatory activities.1–3 In previous research, a sesquiterpene coumarin, kellerin, a major constituent of Ferula sinkiangensis K. M. Shen, has been proved to be a potential natural therapeutic agent for Alzheimer's disease (AD) by inhibition of the inflammatory cytokines nitric oxide (NO), tumor necrosis factor-α (TNF-α), cyclooxygenase-2 (COX-2), interleukin-6 (IL-6) and interleukin-1β (IL-1β) in over-activated BV2 mouse microglial cells.4 However, it is difficult to synthesize and modify the bioactive natural product by chemical methods because of its multiple chiral centers and the chemical instabilities of the skeleton. Therefore, in this research, biotransformation was used in anticipation of producing more derivatives of kellerin more conveniently.Biotransformation produces chemical transformation in enzymes, plant cells or microorganisms' systems,5 and it has become an economically and ecologically competitive technology for modifying chemical compounds, especially for bioactive natural compounds with complex structures.6,7 The plant cultured cells can modify natural products with the advantages of stereo selectivity, mild conditions and diverse products when compared with chemical synthesis.8–10 Angelica sinensis (Oliv.) Diels (A. sinensis) is a traditional medicinal plant, rich in phenylpropanoids, phthalides, terpenoids, essential oils and aromatic compounds.11,12 Its abundant biosynthetic enzymes of phenylpropanoids and terpenoids have attracted the attention of researchers. Preliminary results obtained using the callus of A. sinensis showed that it was able to transform kellerin successfully. Therefore, it was chosen for use in producing derivatives of kellerin.
As far as is known, this is the first time the biotransformation of the natural sesquiterpene, coumarin, has been performed using A. sinensis callus. As a result, 14 products (1–14) were isolated and identified from the suspension cultures of A. sinensis callus. Next, the possible biosynthetic pathways of compounds 1–8 from kellerin were proposed. Furthermore, the primary transformation rules of sesquiterpene, coumarin, skeleton in A. sinensis callus, which fill the gaps not only in the study of the biotransformation of the sesquiterpene, coumarin but also on the application of A. sinensis callus were also considered. Finally, the neuro-inflammation inhibitory activities of kellerin and its transformed products were evaluated using NO assays in lipopolysaccharide (LPS)-induced BV2 microglial cells.13
Results and discussion
Identification of transformed products
A large number of assays were carried out to optimize the induction of A. sinensis callus and subculture conditions by varying the composition of callus induction media, plant organs and callus type, and large-scale tissue culture of A. sinensis callus was obtained.14 Kellerin (855 mg) was distributed into A. sinensis callus and they were co-incubated for one week under the optimal subculture conditions. After incubation, mixtures were separated into the cultures and medium, then they were further extracted and purified using repeated chromatographic and recrystallization methods.As a result, 14 products were obtained, including four new sesquiterpene coumarin derivatives (1–4) together with 10 known ones (5–14). Their structures were identified as: 14′-hydroxy-(3′S,4′R,5′S,8′R,9′S,10′R)-kellerin (1), 5′,6′-ene-14′-hydroxy-(3′S,4′R,8′R,9′S,10′R)-kellerin (2), 5′,6′-ene-(3′R,8′R,9′S,10′R)-ferukrin (3), 14′-hydroxy-(3′S,4′R,5′S,8′R,9′S,10′R)-deacetylkellerin (4), (3′S,5′S,8′R,9′S,10′R)-deacetylkellerin (5),15 ferukrin (6),16 gummosin (7),17 7-hydroxycoumarin (8),18 ferulic acid (9),19 bis(2-ethylhexyl)phthalate (10),20 3-heptyl-3-hydroxy-2,4(1H, 3H)-quinolinediones (11),21 7-methoxy-1-methyl-9H-pyrido[3,4-b]indole (12),22 methyl butyrate (13),23 (Z)-3-methyl-4-oxobut-2-enoic acid (14)24 (Fig. 1). Among them, compounds 1–8 were derivatives of kellerin, whereas compounds 11, 12 and 14 were produced by the culture medium stimulated by the substrate kellerin.11,12
Compound 1 was obtained as a yellow oil (methanol; MeOH), [α]20D + 29.6 (c 1.0, MeOH), high-resolution electrospray ionisation mass spectroscopy (HR-ESI-MS) showed a pseudo molecular ion peak at m/z 481.2196 [M + Na]+ (calcd 481.2197 for C26H34O7Na), which indicated the molecular formula of 1 was C26H34O7. The proton-nuclear magnetic resonance (1H-NMR) and 13C-NMR spectra of compound 1 closely matched those of kellerin except for the absence of signals for a tertiary methyl group and the presence of resonance for a hydroxymethyl group in 1.4 The 1H-NMR spectrum of 1 exhibited typical signals for 7-O-substituted coumarin at δH 7.90 (1H, d, J = 9.5 Hz, H-4), 7.57 (1H, d, J = 8.0 Hz, H-5), 6.97 (1H, d, J = 8.0, 2.0 Hz, H-6), 6.99 (1H, d, J = 2.0 Hz, H-8) and δH 6.25 (1H, d, J = 9.5 Hz, H-3). However, the existence of an oxygenated methylene group and a hydroxymethyl unit can also be predicted by the signals at δH 4.29 (1H, d, J = 10.9, 2.3 Hz, H-11′a), 4.22 (1H, d, J = 10.9, 3.0 Hz, H-11′b), 3.82 (1H, d, J = 11.5 Hz, H-14′a), 3.42 (1H, d, J = 11.5 Hz, H-14′b). Furthermore, four methyl groups were suggested according to the signals observed at δH 1.01 (3H, s, Me-14′), 1.29 (3H, s, Me-12′), 1.34 (3H, s, Me-15′) and δH 1.72 (3H, s, Me-17′). Then 26 carbon signals were displayed in the 13C-NMR spectrum of 1, which indicated a typical sesquiterpene coumarin skeleton including 9 carbons for umbelliferone, 15 which were ascribed to a sesquiterpene moiety and 2 for an acetyl group. Most importantly, the spectral data of one-dimensional (1D) NMR (Table 1) suggested 1 to be a kellerin derivative.
| Position | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| C | H | C | H | C | H | C | H | |
| 2 | 163.8 | 163.8 | 163.8 | 163.8 | ||||
| 3 | 113.5 | 6.25 (1H, d, J = 9.5 Hz) | 113.4 | 6.25 (1H, d, J = 9.5 Hz) | 113.4 | 6.25 (1H, d, J = 9.5 Hz) | 113.1 | 6.24 (1H, d, J = 9.5 Hz) |
| 4 | 145.7 | 7.90 (1H, d, J = 9.5 Hz) | 145.6 | 7.89 (1H, d, J = 9.5 Hz) | 145.6 | 7.90 (1H, d, J = 9.5 Hz) | 145.7 | 7.88 (1H, d, J = 9.5 Hz) |
| 5 | 130.7 | 7.57 (1H, d, J = 8.0 Hz) | 130.8 | 7.57 (1H, d, J = 8.0 Hz) | 130.8 | 7.56 (1H, d, J = 8.0 Hz) | 130.4 | 7.53 (1H, d, J = 8.0 Hz) |
| 6 | 114.3 | 6.99 (1H, dd, J = 8.0, 2.0 Hz) | 114.4 | 6.99 (1H, dd, J = 8.0, 2.0 Hz) | 114.4 | 6.93 (1H, dd, J = 8.0, 2.0 Hz) | 114.1 | 6.95 (1H, dd, J = 8.0, 2.0 Hz) |
| 7 | 163.6 | 163.7 | 163.7 | 163.6 | 6.96 (1H, d, J = 2.0 Hz) | |||
| 8 | 102.0 | 6.97 (1H, d, J = 2.0 Hz) | 102.1 | 6.97 (1H, d, J = 2.0 Hz) | 102.1 | 6.99 (1H, d, J = 2.0 Hz) | 102.2 | |
| 9 | 157.4 | 157.5 | 157.5 | 157.4 | ||||
| 10 | 114.4 | 114.5 | 114.5 | 114.4 | ||||
| 1′ | 31.6 | 1.80 (1H, d, J = 12.8 Hz) | 31.7 | 1.80 (1H, d, J = 12.8 Hz) | 31.7 | 1.80 (1H, d, J = 12.8 Hz) | 31.6 | 1.80 (1H, d, J = 12.8 Hz) |
| 1.13 (1H, t, J = 3.2 Hz) | 1.13 (1H, t, J = 3.2 Hz) | 1.13 (1H, t, J = 3.2 Hz) | 1.13 (1H, t, J = 3.2 Hz) | |||||
| 2′ | 23.7 | 2.05 (1H, m)/1.57 (1H, m) | 23.9 | 2.05 (1H, m)/1.57 (1H, m) | 23.9 | 2.05 (1H, m)/1.57 (1H, m) | 23.7 | 2.05 (1H, m)/1.57 (1H, m) |
| 3′ | 75.8 | 5.07 (1H, t, J = 2.7 Hz) | 75.8 | 5.33 (1H, m) | 79.7 | 3.15 (1H, m) | 71.5 | 3.35 (1H, t, J = 2.7 Hz) |
| 4′ | 39.0 | 39.0 | 39.0 | 41.5 | ||||
| 5′ | 45.7 | 2.16 (1H, m) | 144.4 | 148.3 | 37.1 | 2.16 (1H, m) | ||
| 6′ | 20.2 | 1.75 (1H, m), 1.45 (1H, m) | 121.8 | 5.27 (1H, m) | 130.0 | 5.83 (1H, m) | 20.2 | 1.75 (1H, m), 1.45 (1H, m) |
| 7′ | 40.7 | 1.76 (2H, m) | 45.1 | 1.76 (2H, m) | 45.1 | 1.76 (2H, m) | 40.7 | 1.76 (2H, m) |
| 8′ | 74.1 | 74.1 | 74.1 | 74.1 | ||||
| 9′ | 59.0 | 1.55 (1H, m) | 59.5 | 1.55 (1H, m) | 59.5 | 1.55 (1H, m) | 59.0 | 1.55 (1H, m) |
| 10′ | 43.7 | 43.6 | 43.6 | 43.7 | ||||
| 11′ | 68.9 | 4.29 (1H, dd, J = 10.9, 2.3 Hz) | 69.0 | 4.34 (1H, dd, J = 10.9, 2.3 Hz) | 69.0 | 4.34 (1H, dd, J = 10.9, 2.3 Hz) | 69.4 | 4.29 (1H, dd, J = 10.9, 2.3 Hz) |
| 4.22 (1H, dd, J = 10.9, 3.0 Hz) | 4.28 (1H, dd, J = 10.9, 3.0 Hz) | 4.28 (1H, dd, J = 10.9, 3.0 Hz) | 4.23 (1H, dd, J = 10.9, 3.0 Hz) | |||||
| 12′ | 32.0 | 1.29 (3H, s) | 31.0 | 1.24 (3H, s) | 29.7 | 1.29 (3H, s) | 32.0 | 1.29 (3H, s) |
| 13′ | 23.0 | 1.01 (3H, s) | 23.0 | 1.06 (3H, s) | 16.0 | 1.07 (3H, s) | 17.8 | 0.75 (3H, s) |
| 14′ | 65.0 | 3.82 (1H, d, J = 11.5 Hz) | 64.7 | 3.64 (1H, d, J = 11.5 Hz) | 27.1 | 1.17 (3H, s) | 77.4 | 3.64 (1H, d, J = 11.5 Hz) |
| 3.42 (1H, d, J = 11.5 Hz) | 3.42 (1H, d, J = 11.5 Hz) | 3.59 (1H, d, J = 11.5 Hz) | ||||||
| 15′ | 25.5 | 1.34 (3H, s) | 25.6 | 1.32 (3H, s) | 31.0 | 1.32 (3H, s) | 25.5 | 1.34 (3H, s) |
| 16′ | 172.6 | 172.7 | ||||||
| 17′ | 19.2 | 1.72 (3H, s) | 19.4 | 1.77 (3H, s) | ||||
In the heteronuclear multiple bond correlation (HMBC) experiment (Fig. 2), the long range correlations from δH 4.25 (2H, CH2-11′) to δC 163.6 (C-7), 74.1 (C-8′), 59.0 (C-9′) and δC 43.7 (C-10′) were observed to construct the C-11′–O–C-7 bridge between sesquiterpene and the coumarin moieties. The cross peaks originating from δH 1.34 (Me-15′) to δC 45.7 (C-5′), 31.6 (C-1′), 59.0 (C-9′) fixed Me-15′ at C-10′. Furthermore, the HMBC correlations from the proton signals at δH 3.82 (1H, H-14′a), 3.42 (1H, H-14′b), 1.01 (Me-13′) to the carbon resonance at δC 75.8 (C-3′), 39.0 (C-4′) located the acetyl group at C-3′. Furthermore, the remaining methyl group Me-12′ was assigned to C-8′ because of the HMBC correlation between δH 1.29 (3H, s, Me-12′) and δC 40.7 (C-7′), 59.0 (C-9′), 74.1 (C-8′). Consequently, the planar structure of compound 1 was determined by 1D and two-dimensional NMR. Allowing for the biosynthesis pathway, the absolute configuration of C-3, C-5, C-8, C-9 and C-10 in compound 1 was identified to be same as for the substrate 3′S,5′S,8′R,9′S,10′R-kellerin. The relative configuration of the chiral carbon atom C-4′ was unambiguously determined by nuclear Overhauser spectroscopy (NOESY) (Fig. 3) correlations from Me-13′ to H-3′, H-5′, H-11′, and from Me-15′ to CH2-14′ and Me-12′.
The absolute configuration was further established by comparison of experimental and calculated electronic circular dichroism (ECD) spectra using time dependent density functional theory (TDDFT).25 A conformational search using the Discovery Studio 3.0 program using a systematic method revealed preferred conformers within a 20 kcal mol−1 energy threshold. These conformers were subjected to geometrical optimization and energy calculation with the Becke, three-parameter, Lee–Yang–Parr (B3LYP) function and 6-31G (d) combined with calculation of vibrational modes to confirm these minima. No imaginary frequencies were found. Calculation of the ECD spectra for these conformers were performed using TDDFT at the B3LYP/6-311+G (2d, p) level. The weighted ECD spectrum in MeOH is shown in Fig. 4A. The calculated ECD spectrum of (3′S,4′R,5′S,8′R,9′S,10′R)-1 closely matched the experimental data, in particular there was a positive Cotton effect (CE) in the 200–220 nm region, and a negative CE in the region of 300–350 nm. Differences between the calculated and experimental spectra were apparently because of minor differences between the calculated and solution conformers of this flexible molecule. Most importantly, the structure of compound 1 was finally determined to be 14′-hydroxy-(3′S,4′R,5′S,8′R,9′S,10′R)-kellerin.
Compound 2 was obtained as yellow solid (MeOH), [α]20D − 24.0 (c 1.0, MeOH), and had a molecular formula of C26H32O7, as established by an HR-ESI-MS quasi-molecular ion peak at m/z 479.2054 [M + Na]+ (calcd 479.2040 for C26H32O7Na). The 1H-NMR spectrum of 2 also exhibited typical signals of 7-O-substituted coumarin at δH 7.89 (1H, d, J = 9.5 Hz, H-4), 7.57 (1H, d, J = 8.0 Hz, H-5), 6.97 (1H, d, J = 8.0, 2.0 Hz, H-6), 6.99 (1H, d, J = 2.0 Hz, H-8) and δH 6.25 (1H, d, J = 9.5 Hz, H-3). However, the proton signals at δH 4.34 (1H, d, J = 10.9, 2.3 Hz, H-11′a) and 4.28 (1H, d, J = 10.9, 3.0 Hz, H-11′b) for an oxygenated methylene group, δH 3.64 (1H, d, J = 11.5 Hz, H-14′a) and δH 3.42 (1H, d, J = 11.5 Hz, H-14′b) for a hydroxymethyl unit, respectively, were found. It also displayed signals for four methyl groups at δH 1.06 (3H, s, Me-13′), 1.24 (3H, s, Me-12′), 1.32 (3H, s, Me-15′) and δH 1.77 (3H, s, Me-17′). Then 26 carbon signals were displayed in the 13C-NMR spectrum, which indicated a sesquiterpene coumarin skeleton including umbelliferone with 9 carbons and a sesquiterpene moiety with 15 carbons. The 1D NMR data of 2 revealed similar structural features to those found in 1, with the major differences being the presence of a double bond at C-5′ and C-6′ in 2, which was proved by resonances at δH 5.27 (1H, m, H-6′) and δC 144.4 (C-5′), 121.8 (C-6′). Most importantly, the spectral data of 1D NMR (Table 1) suggested that 2 was a kellerin analog.
In Fig. 2, the HMBC correlations from δH 4.31 (2H, CH2-11′) to δC 163.7 (C-7), 74.1 (C-8′), 59.5 (C-9′) and δC 43.6 (C-10′) were verified by the presence of the C-11′–O–C-7 bridge between sesquiterpene and coumarin moieties. The long range interactions of δH 1.32 (Me-15′) and δC 144.4 (C-5′), 31.7 (C-1′) fixed Me-15′ at C-10′. Furthermore, correlations from the proton signals at δH 3.64 (1H, H-14′a), 3.42 (1H, H-14′b), 1.06 (Me-13′) to the carbon resonances at δC 75.8 (C-3′), 39.0 (C-4′) proved that a hydroxymethyl group and Me-13′ were connected at C-4′. Furthermore, the remaining methyl group Me-12′ was unambiguously assigned to C-8′ because of the HMBC correlations between the proton signals at δH 1.24 (Me-12′) and the carbon resonances at δC 45.1 (C-7′), 59.5 (C-9′) and 74.1 (C-8′). According to the biosynthesis pathway, it can be verified that the hydroxymethyl group was α-oriented, and the absolute configuration of 2 was identified to be the same as the substrate kellerin, that is 3′S, 4′R, 8′R, 9′S, 10′R. Most importantly, the structure of compound 2 was finally determined to be 5′,6′-ene-14′-hydroxy-(3′S,4′R,8′R,9′S,10′R)-kellerin.
Compound 3, a white powder, [α]20D − 23.7 (c 1.0, MeOH), had a molecular formula of C24H28O5 based on a quasi-molecular ion peak at m/z 419.2 [M + Na]+ (calcd 419.2 for C24H28O5Na) in ESI-MS that required 10 indices of hydrogen deficiency. The 1H-NMR and 13C-NMR data of 3 were superimposable with those of kellerin, with the exception of slight changes such as the absence of acetyl group signals and the appearance of resonances of olefin. The 1H-NMR spectrum of 3 showed the signals for a typical 7-O-substituted coumarin at δH 7.90 (1H, d, J = 9.5 Hz, H-4), 7.56 (1H, d, J = 8.0 Hz, H-5), 6.93 (1H, d, J = 8.0, 2.0 Hz, H-6), 6.55 (1H, d, J = 2.0 Hz, H-8) and δH 6.25 (1H, d, J = 9.5 Hz, H-3). However, the resonances of olefin at δH 5.83 (1H, m, H-6′) in the downfield and the proton signals at δH 4.34 (1H, d, J = 10.9, 2.3 Hz, H-11′a) and 4.28 (1H, d, J = 10.9, 3.0 Hz, H-11′b) for an oxygenated methylene group can be observed. Furthermore, according to the signals observed at δH 1.07 (3H, s, Me-14′), 1.17 (3H, s, Me-13′), 1.29 (3H, s, Me-12′) and δH 1.32 (3H, s, Me-15′) showed four methyl groups. Then 24 carbon signals were exhibited in the 13C-NMR spectrum, which indicated a typical sesquiterpene coumarin skeleton including 9 carbons for umbelliferone, and 15 carbons which could be ascribed to a sesquiterpene moiety. So the spectra data of 1D NMR (Table 1) suggested 3 to be a sesquiterpene coumarin.
In the HMBC spectrum (Fig. 2), the correlations between δH 4.31 (2H, CH2-11′) and δC 163.6 (C-7), 74.1 (C-8′), 59.0 (C-9′), 43.7 (C-10′) indicated the presence of the C-11′–O–C-7 bridge between the sesquiterpene and coumarin moieties. The HMBC correlations from δH 1.32 (Me-15′) to δC 148.3 (C-5′) and δC 31.7 (C-1′) were observed, and this suggested that Me-15′ was connected to C-10′. Furthermore, a hydroxyl group was located at C-3′, the presence of which was supported by the HMBC correlations from the proton signals at δH 1.17 (Me-13′), 1.06 (Me-14′) to the carbon resonance at δC 79.7 (C-3′), 39.0 (C-4′), 148.3 (C-5′). Me-12′ was assigned to C-8′ because of the HMBC correlations from δH 1.29 (Me-12′) to δC 45.1 (C-7′), 59.5 (C-9′) and δC 74.1 (C-8′). However, the absolute configuration of C-3′, the chiral center of compound 3, was elucidated by comparing the data of H-3′ and C-3′ obtained from the 1H-NMR and 13C-NMR experiments. With deuterated methanol (CD3OD) as the deuterated reagent, the proton signals at δH 3.50–3.30 (H-3′) and the carbon resonance at δC 77.0–71.0 (C-3′) indicated that the hydroxyl group was β-oriented (3′S), whereas at δH 3.25–3.10 (H-3′) the carbon resonance at δC 80.0–79.0 (C-3′) was α-oriented (3′R).4 Thus, the orientation of the hydroxyl group was α-oriented with δH 3.15 (H-3′) and δC 79.7 (C-3′). The absolute configuration of 3 was identified to be 3′S, 8′R, 9′S, 10′R. Most importantly, the structure of compound 3 was finally determined to be 5′,6′-ene-(3′S,8′R,9′S,10′R)-ferukrin.
Compound 4 was isolated as a yellow oil, [α]20D − 26.2 (c 1.13, MeOH), HR-ESI-MS showed a quasi-molecular ion peak at m/z 439.2092 [M + Na]+ (calcd 439.2091 for C24H32O6Na), which indicated that the molecular formula was C24H32O6. The 1H-NMR spectrum of 4 exhibited resonances for a typical 7-O-substituted coumarin at δH 7.88 (1H, d, J = 9.5 Hz, H-4), 7.53 (1H, d, J = 8.0 Hz, H-5), 6.95 (1H, d, J = 8.0, 2.0 Hz, H-6), 6.96 (1H, d, J = 2.0 Hz, H-8) and 6.24 (1H, d, J = 9.5 Hz, H-3). However, an oxygenated methylene group at δH 4.29 (1H, d, J = 10.9, 2.3 Hz, H-11′a), 4.23 (1H, d, J = 10.9, 3.0 Hz, H-11′b), a hydroxymethyl unit at δH 3.64 (1H, d, J = 11.5 Hz, H-14′a), 3.59 (1H, d, J = 11.5 Hz, H-14′b) appeared in the 1H-NMR. Furthermore, three methyl groups were displayed at δH 0.75 (3H, s, Me-13′), 1.29 (3H, s, Me-12′) and δH 1.34 (3H, s, Me-15′). Then 9 carbons for umbelliferone and 15 carbons ascribed to a sesquiterpene moiety indicated a typical sesquiterpene coumarin skeleton (Table 1).
The C-11′–O–C-7 bridge between the sesquiterpene and coumarin moieties was supported by the long range HMBC correlations from δH 4.26 (2H, CH2-11′) to δC 163.6 (C-7), 74.1 (C-8′), 59.0 (C-9′) and δC 43.7 (C-10′) in Fig. 2. The correlations between δH 1.34 (Me-15′) and δC 37.1 (C-5′), 31.6 (C-1′) revealed a tertiary methyl Me-15′ was fixed at C-10′. Then the HMBC interactions from the proton signals at δH 3.64 (1H, H-14′a), 3.59 (1H, H-14′b), 0.75 (Me-13′) to the carbon resonances at δC 71.5 (C-3′), 41.5 (C-4′), 37.1 (C-5′) were proved to be from the location of C-3′ with a β-oriented hydroxyl group. Furthermore, the residual index Me-12′ was assigned to C-8′ because of the HMBC correlations between the proton signals at δH 1.29 (3H, s, Me-12′) and the carbon resonances at δC 40.7 (C-7′), 59.0 (C-9′) and 74.1 (C-8′). Most importantly, the structural elucidation of compound 4, which was straightforward, was determined to be 14′-hydroxy-(3′S,4′R,5′S,8′R,9′S,10′R)-deacetylkellerin.
By comparison of experimental and calculated ECD spectra, the absolute configuration of compound 4 was further established and is shown in Fig. 4B. The calculated ECD spectrum of (3′S,4′R,5′S,8′R,9′S,10′R)-4 closely matched the experimental data, in particular a negative CE in the 200–220 nm and 270–350 nm regions, and a positive CE in the region of 220–270 nm. Differences between calculated and experimental spectra were apparent because of minor differences between the calculated and solution conformers of this flexible molecule. Thus, the absolute configuration of 4 was established as 3′S, 4′R, 5′S, 8′R, 9′S, 10′R.
Proposed biotransformation pathway of products
The possible metabolic pathways of the substrate are outlined in Fig. 5. Firstly, kellerin was transformed into compound 1 by hydroxylation at Me-14′. Then the linkage between the coumarin and sesquiterpene moieties were broken to yield 8, 7-hydroxycoumarin. Compound 5 was produced from kellerin through deacetylation. Then 5 was dehydrated at C-3′ to produce the intermediate 5a. Compound 6 was produced by the addition of a double bond at C-2′, 3′ in 5a. After that, compound 5 was further dehydrated at C-8′, and C-12′ to give compound 7. In addition, compound 5 was hydroxylated at Me-14′ to give compound 4. Compound 6 was converted to generate compound 3 via dehydrogenation of C-5′, and C-6′. The formation of compound 2 was also involved in the dehydrogenation at C-5′, C-6′ of 1.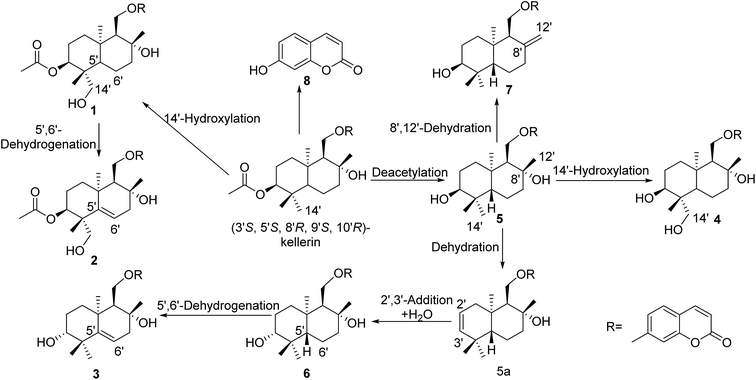 | ||
| Fig. 5 Possible biosynthesis pathways of compounds 1–8 from kellerin in Angelica sinensis (Oliv.) Diels callus. | ||
Furthermore, compounds 11, 12, 14 were produced using a culture medium stimulated by kellerin, as shown in Fig. 6. Aniline and 2-heptylmalonic acid were condensed to generate the intermediate 11a, which was transformed to compound 11 using addition and oxidation. Harmane was converted into compound 12 using hydroxylation and methylation reactions. Compound 14 was derived from 5-hydroxy-4-methylfuran-2(5H)-one using a cycloreversion reaction and oxidation process. Compounds 9, 10 and 13 were derived from A. sinensis callus.
 | ||
| Fig. 6 Possible biosynthesis pathways of compounds 11–12, 14 in Angelica sinensis (Oliv.) Diels callus stimulated by kellerin. | ||
It is the first time that the biotransformation of sesquiterpene coumarins using the A. sinensis callus system has been explored. Next, using the characteristics of the transformed products, a brief summary of the method using A. sinensis callus to obtain sesquiterpene coumarins will be given. Firstly, the sesquiterpene moiety of the substrate was susceptible to being modified, which involved hydroxylation, dehydrogenation, dehydration and reduction reactions. Secondly, the ether linkage (C-11′–O–C-7 bridge) between sesquiterpene and the coumarin moieties was readily fractured and this gave coumarin. Thirdly, the products, produced by the culture medium stimulated by the substrate, were usually obtained from oxidation, reduction and methylation processes.
Anti-neuroinflammatory effects of transformed products in LPS-induced BV2 microglial cells
The anti-inflammatory activities of the identified products were evaluated using a NO assay in LPS-induced BV2 microglial cells. However, the cytotoxic activities were assayed to avoid possible effects of reduced viability on NO release (Fig. 1S; ESI†). The n-butanol (n-BuOH) extract (BE) showed anti-inflammatory activities with an IC50 value (the concentration of an inhibitor where the response (or binding) is reduced by half) of 19.53 μg mL−1 (Table 2). The ethyl acetate (EtOAc) extract (EE) could reduce the production of NO significantly at a concentration of 100 μg mL−1. Compounds 1, 2, 3, 6, 7 and 9 exhibited stronger inhibitory activities on NO production than that of the positive control minocycline. Out of these compounds, 1 and 7 exhibited significant inhibitory effects with an IC50 value of 7.55, at a concentration of 24.47 μM. Compounds 2, 3, 6, 9 could reduce the production of NO significantly with IC50 values ranging from 3.72 μM to 35.46 μM (Table 2). Compounds 5, 8, 13 exhibited activities with IC50 values ranging from 47.34 μM to 74.31 μM (Fig. 7A and B and Table 2). In addition, compounds 4, 11 and 14 showed a trend of inhibition on NO production. It is worth noting that all the samples tested, except for compound 10 did not exhibit obvious cytotoxicities at their effective concentrations (Fig. 1S; ESI†).| Sample name | IC50a | Sample name | IC50a |
|---|---|---|---|
| a IC50 (μg mL−1 for extracts and μM for compounds).b Compounds 1, 7, 10 showed cytotoxicity at 100 μM.c Positive control.d SEM = standard error of the mean. | |||
| EE | >100 | Compound 8 | 74.31 ± 4.50 |
| BE | 19.53 ± 3.54 | Compound 9 | 24.64 ± 4.33 |
| Compound 1b | 7.55 ± 1.97 | Compound 10b | 61.52 ± 3.51 |
| Compound 2 | 21.62 ± 3.30 | Compound 11 | >100 |
| Compound 3 | 24.89 ± 2.67 | Compound 12 | >100 |
| Compound 4 | >100 | Compound 13 | 64.41 ± 3.98 |
| Compound 5 | 47.34 ± 3.77 | Compound 14 | >100 |
| Compound 6 | 35.46 ± 2.74 | Substrate | 3.72 ± 2.02 |
| Compound 7b | 24.47 ± 3.71 | minocyclinec | 35.82 ± 3.60 |
According to the biological results and reported structure–activity relationships (SAR) in previous research,4 the SAR of sesquiterpene coumarins were further enriched as shown in Fig. 8. Firstly, as for the anti-neuroinflammatory effects of bicyclic sesquiterpene coumarins, with a terminal double bond substituted at C-8′, the order is β-OH, acetoxyl group, carbonyl group, and α-OH (Fig. 8A). Secondly, the effects of sesquiterpene coumarins with the presence of methyl and hydroxyl groups at C-8′, depended on the substitution at C-3′. Following the sequential order of acetoxyl group, α-OH, β-OH, and carbonyl group at C-3′, neuroinflammation inhibitory activities decreased (Fig. 8B). Thirdly, the presence of the C-14′-methyl was favorable to anti-inflammatory activity, although the hydroxylation of C-14′ decreased the effect, for example, with compound 1 versus substrate (IC50 of 7.55 μM versus 3.72 μM). Finally, compounds with a double group present at Δ5′,6′, for example, compounds 1 and 2, showed a dramatic decrease of activity, which indicated that the saturated β-ring in the bicyclic skeleton of sesquiterpene coumarins was critical for bioactivity.
The possible anti-inflammatory mechanism for kellerin derivatives was likely to be similar to that of kellerin. In previous work, the primary mechanism of kellerin was tested by using a quantitative real-time polymerase chain reaction (qRT-PCR). The results showed that kellerin could significantly inhibit the mRNA expression of inflammatory factors TNF-α, IL-6, IL-1β and COX-2 induced by LPS in BV2 microglial cells at concentrations of 1–10 μM.4 Therefore, the kellerin derivatives described in this paper might also exhibit their effect by inhibiting the release of inflammatory cytokines in over-activated microglials. Thus, further research will be done to investigate the anti-inflammatory mechanism of the kellerin derivatives identified in this research.
Experimental
General experimental procedures
NMR spectra were recorded on an AVANCE III HD 600 MHz instrument (Bruker, Switzerland), with trimethylsilane (TMS) as an internal standard. The chemical shifts were measured relative to TMS and expressed in δ (ppm) and the coupling constants (J) are reported in Hertz (Hz). A quadropole time-of-flight mass spectrometer (Q-TOF-MS): micrOTOF-Q mass spectrometer (Bruker), was used to collect HR-ESI-MS data, in the m/z (rel.%) mode. Optical rotations were detected using a modular circular polarimeter: MCP 200 Polarimeter (Anton Paar). Silica gel (SiO2: 50–74 μm) for chromatography was obtained from the Qingdao Ocean Chemical Group Co. (China). Sephadex LH-20 was purchased from Pharmacia (Switzerland). Octadecylsilyl (ODS; 100–50 μm) chromatographic columns were purchased from YMC (Japan). High performance chromatography (HPLC) separation was carried out on a ODS-A C18 column (5 μm, 250 mm × 20 mm) equipped with a SPD-20A ultraviolet detector (Shimadzu) and a LC-20AR series pumping system (Shimadzu). Dimethyl sulfoxide (DMSO), deuterated DMSO (DMSO-d6) and CD3OD were obtained from the Sigma-Aldrich Company (St. Louis, MO, USA). All reagents were chromatographic grade or analytical grade and were obtained from Tianjin DaMao Chemical Company (Tianjin, China).Substrate and culture system
Kellerin was isolated from the chloroform extract of the gum resin of F. sinkiangensis and was authenticated by comparing its physical and spectroscopic data to reported values.4 Its purity was determined to be 99.8% by HPLC analysis.Petiole I (the portion adjacent to the leaf) of A. sinensis was collected from the South China Botanical Garden (Guangzhou, China), as explant material. Huang et al. induced the A. sinensis callus according to the optimized composition of callus induction media, plant organs, and callus type reported in previous work.14 Tissue culture conditions for A. sinensis were optimized using leaves and petioles (types I and II) as explant sources and petiole I was found to be the best plant organ for callus induction.
Petiole I was placed in running tap water for 30 min to wash away dust and other particles, then surface sterilized for 1 min by soaking in 75% (v/v) ethanol, then completely sterilized for 8–10 min in a 0.1% (w/v) aqueous solution of mercuric chloride, and washed five times in sterilized water (H2O). Then petiole I was cut into approximately 1.5 cm segments, and cultured on Murashige and Skoog medium in 100 mL conical flasks, supplemented with 5 mg L−1 of α-naphthaleneacetic acid (NAA), 0.5 mg L−1 of 6-benzyladenine (BA), 0.7 mg L−1 of 2,4-dichlorophenoxy acetic acid (2,4-D), 30 g L−1 of sucrose, and 7.5 g L−1 of agar, for induction of primary calli in a growth room at 25 °C ± 1 °C under complete darkness.14 After that, the primary calli were sub-cultured in 250 mL Erlenmeyer flasks containing Gamborg's B-5 medium with 1.0 mg L−1 2,4-D, 0.2 mg L−1 of BA and 30 g L−1 of sucrose at 25 °C ± 1 °C under darkness every three weeks.
Culture conditions and biotransformation procedures
The fresh primary A. sinensis callus was cultured on the B-5 liquid medium [15 g L−1 sucrose (w/v)] with NAA (1.0 mg L−1), 2,4-D (0.5 mg L−1), and kinetin (0.05 mg L−1). The pH value of the medium was adjusted to 5.75. Callus cells (6 g) were inoculated into a 250 mL Erlenmeyer flask with 120 mL B-5 medium, and then left under complete darkness at 25 °C ± 1 °C on a rotary shaker at 110 rpm for 20 days.Kellerin (855 mg) was dissolved in MeOH (46.0 mL) and distributed equally into 57 bottles of the suspension cultures of A. sinensis callus. The control groups had the same concentration of kellerin but the flasks only contained the culture medium without cells or by just adding the same volume of MeOH to suspension cultured cells without substrate. All of the flasks were incubated under the same conditions throughout the experiment for 7 days at 25 °C ± 1 °C in the dark.
Extraction and isolation of biotransformation products
After incubation, the mixtures were filtered to separate the cultures and the medium. Then the medium was concentrated to 200 mL by evaporation in vacuo and extracted using the same volume of EtOAc and n-BuOH successively for three times to obtain Fr. 1 (EE; 0.70 g) and Fr. 2 (n-BuOH extract; 0.98 g). The cultures were dried under 55 °C, immersed in MeOH for 12 h and extracted using ultrasonic mixing for 30 min to obtain the MeOH extract (5.30 g), which was then resuspended in water and partitioned using EtOAc or n-BuOH to give Fr. 3 (EE; 0.21 g) and Fr. 4 (n-BuOH extract) (1.12 g), respectively. For the control group, parallel procedures were employed.The yield of kellerin derivatives was calculated according using the formula:
| Yield of product = amount of the product (mg)/[total amount of the substrate (mg) − amount of the substrate (non-transformed)]. |
The EE (0.91 g) of cultures and medium (Fr. 1 and Fr. 3) was combined and then subjected to HPLC on an ODS column with an elution gradient of MeOH/H2O (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–100
100–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) to get five subfractions (Fr. 1a–5a). Next, Fr. 1a was separated using HPLC with an ODS column (250 mm × 10 mm, flow rate 3 mL min−1) eluted with acetonitrile (MeCN)/H2O (37
0) to get five subfractions (Fr. 1a–5a). Next, Fr. 1a was separated using HPLC with an ODS column (250 mm × 10 mm, flow rate 3 mL min−1) eluted with acetonitrile (MeCN)/H2O (37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63) to give compound 11 (2.0 mg, retention time (tR) = 27 min) and compound 12 (1.3 mg, tR = 43 min). Then Fr. 2a was purified using HPLC with an ODS column (250 mm × 10 mm, flow rate 3 mL min−1) to yield compound 8 (1.9 mg, 0.30%, tR = 40 min) and compound 9 (2.3 mg, tR = 56 min) using MeOH/H2O (35
63) to give compound 11 (2.0 mg, retention time (tR) = 27 min) and compound 12 (1.3 mg, tR = 43 min). Then Fr. 2a was purified using HPLC with an ODS column (250 mm × 10 mm, flow rate 3 mL min−1) to yield compound 8 (1.9 mg, 0.30%, tR = 40 min) and compound 9 (2.3 mg, tR = 56 min) using MeOH/H2O (35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65) as the eluting solvent. Fr. 3a was firstly fractionated using silica gel column chromatography (3 × 50 cm) with a gradient elution of petroleum ether/acetone (0
65) as the eluting solvent. Fr. 3a was firstly fractionated using silica gel column chromatography (3 × 50 cm) with a gradient elution of petroleum ether/acetone (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–100
100–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) to get sub-fractions, 3a-1 and 3a-2. Then 3a-1 was purified using HPLC with an ODS column (250 mm × 10 mm, flow rate 3 mL min−1) using MeOH/H2O (85
0) to get sub-fractions, 3a-1 and 3a-2. Then 3a-1 was purified using HPLC with an ODS column (250 mm × 10 mm, flow rate 3 mL min−1) using MeOH/H2O (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) as a mobile phase, and this provided compound 10 (1.5 mg, tR = 43 min) and compound 13 (1.8 mg, tR = 59 min). Compound 14 (1.3 mg, tR = 25 min) was obtained from sub-fraction 3a-2 using HPLC separation with an ODS column (250 mm × 10 mm, flow rate 3 mL min−1) using MeOH/H2O (56
15) as a mobile phase, and this provided compound 10 (1.5 mg, tR = 43 min) and compound 13 (1.8 mg, tR = 59 min). Compound 14 (1.3 mg, tR = 25 min) was obtained from sub-fraction 3a-2 using HPLC separation with an ODS column (250 mm × 10 mm, flow rate 3 mL min−1) using MeOH/H2O (56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44) as eluting solvent. Compound 5 (0.8 mg, 0.13%, tR = 45 min) and compound 6 (1.3 mg, 0.20%, tR = 59 min) were obtained from Fr. 5a using HPLC with an ODS column (250 mm × 10 mm, flow rate 3 mL min−1) eluted with MeCN/H2O (40
44) as eluting solvent. Compound 5 (0.8 mg, 0.13%, tR = 45 min) and compound 6 (1.3 mg, 0.20%, tR = 59 min) were obtained from Fr. 5a using HPLC with an ODS column (250 mm × 10 mm, flow rate 3 mL min−1) eluted with MeCN/H2O (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60). Both the combinations (BE, 2.10 g) of Fr. 2 and Fr. 4 were subjected to HPLC on an ODS column gradient eluted with MeOH/H2O (from 0
60). Both the combinations (BE, 2.10 g) of Fr. 2 and Fr. 4 were subjected to HPLC on an ODS column gradient eluted with MeOH/H2O (from 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 to 100
100 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) to get seven fractions (Fr. 1b–7b). Fr. 1b was isolated using HPLC with an ODS column (250 mm × 10 mm, flow rate 3 mL min−1) with MeOH/H2O (55
0) to get seven fractions (Fr. 1b–7b). Fr. 1b was isolated using HPLC with an ODS column (250 mm × 10 mm, flow rate 3 mL min−1) with MeOH/H2O (55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45) and then purified with Sephadex LH-20 chromatography eluted with MeOH to give compound 1 (2.1 mg, 0.33%) and compound 2 (1.0 mg, 0.16%). Then, compounds 3 (1.2 mg, 0.19%, tR = 53 min) and 4 (1.5 mg, 0.24%, tR = 73 min) were obtained from Fr. 3b using HPLC with an ODS column (250 mm × 10 mm, flow rate 3 mL min−1) eluted with MeOH/H2O (55
45) and then purified with Sephadex LH-20 chromatography eluted with MeOH to give compound 1 (2.1 mg, 0.33%) and compound 2 (1.0 mg, 0.16%). Then, compounds 3 (1.2 mg, 0.19%, tR = 53 min) and 4 (1.5 mg, 0.24%, tR = 73 min) were obtained from Fr. 3b using HPLC with an ODS column (250 mm × 10 mm, flow rate 3 mL min−1) eluted with MeOH/H2O (55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45). Fr. 4b was loaded on a silica gel column (3 × 50 cm) with gradient elution of dichloromethane/MeOH of increasing polarity to get sub-fraction 4b-1 and 4b-2. Then 4b-1 was purified using HPLC with an ODS column (250 mm × 10 mm, flow rate 3 mL min−1) with MeCN/H2O (43
45). Fr. 4b was loaded on a silica gel column (3 × 50 cm) with gradient elution of dichloromethane/MeOH of increasing polarity to get sub-fraction 4b-1 and 4b-2. Then 4b-1 was purified using HPLC with an ODS column (250 mm × 10 mm, flow rate 3 mL min−1) with MeCN/H2O (43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57) to obtain compound 7 (1.5 mg, 0.24%, tR = 19 min) and substrate (220.0 mg, tR = 39 min).
57) to obtain compound 7 (1.5 mg, 0.24%, tR = 19 min) and substrate (220.0 mg, tR = 39 min).
Structure characterization of new compounds
Viability assay, and measurement of NO production
Cell viability was determined using the methylthiazolyldiphenyl-tetrazolium bromide (MTT) reduction assay.8 Briefly, cells were plated on 96-well microtiter plates and pretreated with EE, BE at concentrations of 100 μg mL−1, 30 μg mL−1, 10 μg mL−1, 1 μg mL−1, 0 μg mL−1 and the tested compounds were used at concentrations of 100 μM, 30 μM, 10 μM, 1 μM, 0 μM in the presence of LPS (100 ng mL−1) for 24 h. Compared with the experimental group, the cells without LPS were used as a control group. Next the cells were incubated with MTT (0.25 mg mL−1) for 4 h at 37 °C. The supernatant of the cells was removed and the formazan crystals were prepared as stock solutions in DMSO. The optical densities were read on a plate reader (Bio-Tek, USA) at 490 nm.Determination of NO production was performed by measuring the accumulation of nitrite in the culture supernatant using Griess reagent, as previously described. Cells were seeded into 96-well microtiter plates and then stimulated with LPS (100 ng mL−1) in the presence of EE, BE at concentrations of 100 μg mL−1, 30 μg mL−1, 10 μg mL−1, 1 μg mL−1, 0 μg mL−1 and each tested sample was used at a concentration of 100 μM, 30 μM, 10 μM, 1 μM, 0 μM at 37 °C for 24 h. Compared with the experimental group, cells without LPS were used as a control group. A portion (50 μL) of the culture supernatant fluids was mixed with 50 μL of Griess reagent at room temperature. The absorbance was measured at 540 nm with a plate reader (Bio-Tek, USA) after fifteen minutes.
Conclusions
In this research, it is the first time sesquiterpene coumarins have been biotransformed using A. sinensis callus. It was discovered that the sesquiterpene moiety of the sesquiterpene coumarin skeleton is susceptible to being modified, which involved hydroxylation, dehydrogenation, dehydration and reduction reactions. However, the ether linkage bridged sesquiterpene and coumarin moieties readily fracture. Research is continuing to reveal more biotransformation regulars of the A. sinensis callus.The anti-neuroinflammatory effect assay revealed that the sesquiterpene coumarin skeleton is a potential bioactive molecule for the development of neuroinflammatory inhibitors. Based on previous research, the SAR between sesquiterpene coumarin and neuroinflammation inhibitory activities was further deduced. The anti-inflammation activities are closely related to 3′-acetyl, 3′-α/β-OH and 14′-methyl moieties. It was then found that the IC50 values seem to reduce following the order: 3′-acetyl < 3′-α-OH < 3′-β-OH < 3′-carbonyl in the skeleton with methyl and hydroxyl at 8′ whereas it was found that 3′-β-OH < 3′-acetyl < 3′-carbonyl < 3′-α-OH in the structure with a terminal double bond at 8′. The presence of 14′-methyl and a saturated β-ring may increase the inhibitory activities. The SAR mentioned previously may provide some useful guidance for future design of anti-neuroinflammatory agents with preferred physical–chemical properties.
Acknowledgements
The supporters of this work include the National Natural Science Foundation of China (U1403102, 81173531, 81473330, 81673323), the Shenyang Science and Technology Research Project (F15-199-1-26), the Research Project for Key Laboratory of Liaoning Educational committee (LZ2015067), the Natural Science Foundation of Liaoning Province (2015020732), the Innovation Team Project of Liaoning Province (LT2015027), the Fund for Long-term Training of Young Teachers in Shenyang Pharmaceutical University (ZCJJ2013409), Program for Liaoning Excellent Talents in University (LR2015022).Notes and references
- A. Gliszczyńska and P. E. Brodelius, Phytochem. Rev., 2012, 11, 77–96 CrossRef.
- H. M. Gao and J. S. Hong, Trends Immunol., 2008, 29, 357–365 CrossRef CAS PubMed.
- M. T. Heneka and M. K. O'Banion, J. Neuroimmunol., 2007, 184, 69–91 CrossRef CAS PubMed.
- Y. C. Xing, N. Li, D. Zhou, G. Chen, K. Jiao, W. L. Wang, Y. Y. Si and Y. Hou, Planta Med., 2016 DOI:10.1055/s-0042-109271.
- Y. Simeo and J. V. Sinisterra, Mini-Rev. Org. Chem., 2009, 6, 128–134 CrossRef CAS.
- S. Deng, B. J. Zhang, C. Y. Wang, Y. Tian, J. H. Yao, L. An, S. S. Huang, J. Y. Peng, K. X. Liu and X. C. Ma, Bioorg. Med. Chem. Lett., 2012, 22, 1615–1618 CrossRef CAS PubMed.
- R. N. Patel, Coord. Chem. Rev., 2008, 252, 659–701 CrossRef CAS.
- K. Ishihara, H. Hamada, T. Hirata and N. Nakajima, J. Mol. Catal. B: Enzym., 2003, 23, 145–170 CrossRef CAS.
- K. D. Deepak, S. K. Amit, K. B. S. Cyrus, O. R. Scott and P. O. John, Chem. Res. Toxicol., 2002, 15, 269–299 CrossRef.
- Y. Simeo and J. V. Sinisterra, Mini-Rev. Org. Chem., 2009, 6, 128–134 CrossRef CAS.
- L. Z. Yi, Y. Z. Liang, H. Wu and D. L. Yuan, J. Chromatogr. A, 2009, 1216, 1999–2001 CrossRef PubMed.
- J. P. Ma, Z. B. Guo, L. Jin and Y. D. Li, Chin. J. Nat. Med., 2015, 13, 0241–0249 Search PubMed.
- Y. Hou, C. F. Wu, J. Y. Yang, X. He, X. Y. Bi, L. Yu and T. Guo, Progr. Neuro. Psychopharmacol. Biol. Psychiatr., 2006, 30, 1523–1528 CrossRef CAS PubMed.
- B. Huang, L. J. Han, S. M. Li and C. Y. Yan, Pharmacogn. Mag., 2015, 13, 574–578 Search PubMed.
- Y. E. Perelson, V. I. Sheichenko and Y. E. Sklyar, Khim.-Farm. Zh., 1977, 11, 33–36 Search PubMed.
- M. E. Perelson, Y. E. Sklyar and N. V. Veselovskaya, Khim.-Farm. Zh., 1977, 11, 366–369 Search PubMed.
- J. A. Marco, J. F. Sanz and A. Yuste, Liebigs Ann. Chem., 1991, 9, 929–931 CrossRef.
- J. L. Wang, L. N. Li and L. D. He, Nat. Prod. Res. Dev., 2007, 19, 427–429 CAS.
- X. J. Yang, W. X. Huang and N. L. Wang, Chin. Herb. Med., 2005, 36, 1604–1607 CAS.
- R. J. Chang, C. H. Wang, Q. Zeng, B. Guan, W. D. Zhang and H. Z. Jin, Arch. Pharmacal Res., 2013, 36, 1291–1301 CrossRef CAS PubMed.
- K. Stanislav, K. Antonín, P. Jiří and K. Janez, Heterocycles, 2002, 57, 1659–1682 CrossRef.
- F. Insaf, B. Jalloul, Z. Mansour, B. G. Fatima and B. J. Hichem, J. Enzyme Inhib. Med. Chem., 2015, 30, 371–377 CrossRef PubMed.
- Y. G. Jiao, F. Zhang and T. S. Dibble, J. Phys. Chem. A, 2015, 119, 7282–7292 CrossRef CAS PubMed.
- F. Robert and T. Kiyoshi, Chemosphere, 1999, 38, 973–978 CrossRef.
- N. M. O'Boyle, A. Tenderholt and K. M. Langner, J. Comput. Chem., 2009, 29, 839–845 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The spectroscopic data of known compounds and cell viability results of transformed products. See DOI: 10.1039/c6ra22502k |
| This journal is © The Royal Society of Chemistry 2016 |








