Thickness-dependent bandgap tunable molybdenum disulfide films for optoelectronics†
Abstract
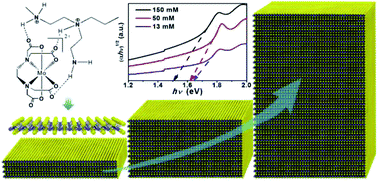
Thickness-dependent bandgap tunable MoS2 has significant potential applications in electronic and optoelectronic devices. Here, we report an aqueous solution approach to grow thickness-controlled MoS2 films. The thicknesses of the MoS2 films can be readily changed from 50 nm to 2.5 nm, corresponding to bandgaps modulated from 1.50 eV to 1.64 eV. Remarkably, MoS2 films with various thicknesses are expediently realized by simple adjustment of the precursor concentration. Microscopic and spectroscopic analyses illustrate that the as-grown MoS2 films are uniform and high quality. Photoresponse tests demonstrate that the as-grown MoS2 films have fast responses to light and good stability.

 Please wait while we load your content...
Please wait while we load your content...