Cu2O hollow structures—microstructural evolution and photocatalytic properties†
Baoshun Wanga,
Weiwei Zhangb,
Zhiyun Zhanga,
Renying Lia,
Yulong Wua,
Zhengguang Hua,
Xiaoling Wua,
Chungang Guoa,
Guoan Chenga and
Ruiting Zheng*a
aKey Laboratory of Radiation Beam Technology and Materials Modification of Ministry of Education, College of Nuclear Science and Technology, Beijing Normal University, Beijing 100875, P. R. China. E-mail: rtzheng@bnu.edu.cn
bSchool of Science, Minzu University of China, Beijing 10081, PR China
First published on 12th October 2016
Abstract
In this paper, Cu2O Single-Shelled Hollow (SSH) sub-micron spheres, Multi-Shelled Hollow (MSH) sub-micron spheres and Multi-Shelled Porous (MSP) sub-micron spheres were successfully synthesized via a facile one-pot route in the presence of hexadecyltrimethylammonium bromide (CTAB) and ascorbic acid (VC). It is found that the morphology and structure of Cu2O hollow nanoparticles can be adjusted by changing alkalinity and aging time. The formation mechanism and structural evolution for these hollow structures is studied. Among three kinds of Cu2O hollow spheres, the Cu2O MSH sub-micron spheres demonstrated the best photo-catalytic (PC) property, which should attribute to the higher light absorption and smaller band gap.
1. Introduction
In recent years, hollow micro-/nanostructures aroused a lot of interest because of their unique properties such as low density, high specific surface area and efficient paths for ion diffusion, which make them have potential applications in energy storage, catalysts, sensors, drug-delivery carriers, photonic crystals, biomedical diagnosis agents, chemical reactors and so on.1–10 In previous researches, different strategies, such as the Kirkendall effect,3,6 template-mediated approaches,11,12 Ostwald ripening,13,14 galvanic replacement,15,16 chemical etching,17–28 water-soluble salt templates have been developed to synthesize hollow structures.19,20As a typical p-type semiconductor, cuprous oxide (Cu2O) has been widely applied in catalysis,21 gas sensors,22 biosensing,23 solar cells,24 photoelectrochemical cells,25 and lithium-ion batteries.26 Lots of progresses have been made in synthesizing different shape of Cu2O hollow structures. Lu and co-workers synthesized octahedral Cu2O hollow nanocages via a catalytic self-templating method.27 Zeng and co-workers firstly synthesized colloidal CuO nanocrystallites, then CuO spherical aggregation and transformed into hollow Cu2O nanospheres.28 Xu et al.29 report the synthesis of multi-shelled hollow Cu2O spheres by the help of vesicle templates. Multi-shelled hollow micro-/nanostructured materials are expected to have better performances over their single-shelled counterparts for applications in catalysis and photocatalysis.30 However, the forming mechanism and their photocatalysis properties of hollow Cu2O spheres still need further investigation.
In this paper, Cu2O MSH, MSP and SSH sub-micron spheres were successfully synthesized via a facile one-pot route in the presence of hexadecyltrimethylammonium bromide (CTAB) and ascorbic acid (VC). We found that CTAB and VC play an important role in the formation of Cu2O hollow spheres. And the structure of Cu2O hollow spheres can be adjusted by simply changing alkalinity and etching time. At last, we made Cu2O PEC devices with three kinds of hollow particles. The experimental results indicate three kinds of Cu2O hollow structures all have good response to light. The photocurrent of Cu2O MSH sub-micron spheres device is higher than the other two. The reason should attribute to its slightly lower band gap and larger absorption range.
2. Experimental section
2.1. The chemicals
Copper(II) sulfate pentahydrate (CuSO4·5H2O, 99%) was purchased from Xilong Chemical Co. Hexadecyltrimethylammonium bromide (C19H42BrN, 99%) and ascorbic acid (C6H8O6, 99.7%) were purchased from Sinopharm Chemical Reagent Co. Sodium hydroxide (NaOH, 98%) was purchased from Beijing Chemical Works. DI water was made in our lab.2.2. Synthesis of Cu2O hollow micro-/nanostructures
Cu2O MSH sub-micron spheres were synthesized by a liquid chemical reaction. In a typical procedure, 3.6436 g of CTAB and 0.05 g of CuSO4·5H2O were put in a beaker with 100 mL de-ionized water, followed by vigorously stirred to obtain solution A. Then 0.18 g of ascorbic acid was added into solution A, and the beaker was immersed in 60 °C of oil bath for 20 min with the speed of 10 rpm to get solution B. After that, 10 mL 0.4 M of NaOH solution was added into above solution B drop by drop under the same rotate speed. The color of the solution B changes from blue to yellow immediately. The solution was stirred for 5 s, and then the precipitate was centrifuged, washed sequentially by de-ionized water and isopropyl alcohol for three times, respectively. After dried in a 60 °C vacuum oven for 5 h, the Cu2O multi-shelled hollow spheres were obtained.Using the similar procedure of Cu2O MSH sub-micron spheres, just prolong reaction time to 10 min or increase the concentration of NaOH to 0.5 M (reaction time is 5 min). The products turn to be Cu2O MSP sub-micron spheres. If we prolong reaction time to 1 h or increase the concentration of NaOH to 2.5 M (reaction time is 5 min). The products turn to be Cu2O SSH sub-micron spheres.
2.3. Structural characterization
The chemical structure and components of as-prepared Cu2O hollow sub-micron spheres were identified by X-ray diffraction (XRD, Bruker D8 Advance diffractometer) with Cu Kα radiation (λ = 0.1506 nm). Morphology and size of the as-prepared Cu2O hollow sub-micron spheres were observed by scanning electron microscopy (SEM, HITACHI S-4800). Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images were obtained by a F20 transmission electron microscopy. Room temperature UV-vis diffuse reflectance spectra were taken on a PerkinElmer Lambda 950 UV-vis spectrophotometer.2.4. Photocurrent measurements
20 mg of as-prepared Cu2O powder was dispersed in 15 mL de-ionized water to make a stable suspension. Then two drops of suspension was dropped onto a piece of FTO glass and dried in a 60 °C thermostat for slowly drying. Copper wires were used to contact the dried thin films. The areas of thin film electrodes were about 1 cm2. Photocurrent measurements were carried out on a bipotentiostat (Modle AFCBP1, USA) in the darkness. The light source employed in PEC activity was a 300 W xenon lamp with an AM 1.5G filter (MAX-302, Asahi Spectra, USA). For all the measurements, three-electrode system was applied, as Fig. S1 shows (see ESI†). The electrolyte was 0.5 M Na2SO4 electrolyte solution (pH = 7) deaerated by bubbling N2 for at least 25 min before each experiment. A platinum line was used as a counter electrode, and Ag/AgCl (in 3.0 M KCl solution) was used as a reference electrode connected to Na2SO4 solution by a salt bridge. Photocurrent measurements were taken from 0 to −1 V vs. Ag/AgCl at a scan rate of 5 mV s−1. All potentials reported in this study are relative to Ag/AgCl (3 M KCl, 0.207 V vs. SHE).3. Results and discussion
3.1. Microstructure of hollow structures
Fig. 1 shows the structures of the three as-prepared products. It can be seen that three kinds of as-prepared products are all sub-micron hollow spheres, but their microstructure are different. Fig. 1(a) is the TEM image of a MSH sphere. It can be observed that the sphere has three layers, the thickness of each layer is about 10 nm. Fig. 1(d) is the SEM image of MSH spheres. The MSH spheres are all closed spheres, the average diameter of spheres is about 150 nm. While we keep other conditions unchanged, just prolong the reaction time to 10 minutes, MSP spheres can be obtained. Fig. 1(b) clearly shows the structure of MSP spheres, we can found that these porous spheres have a multi-shelled structure and the average shell thickness is about 22.55 nm. Fig. 1(e) is the SEM image of MSP spheres. The average diameter of porous sub-micron sphere is about 150–200 nm and the average diameter of the pores on the sphere surface is about 30 nm. As the reaction time further extended to 1 hour, MSP spheres turn into SSH spheres, as shown in Fig. 1(c). TEM image shows that these particles have an obvious empty interior. The average shell thickness of the hollow sphere is about 32.2 nm. SEM image (Fig. 1(f)) indicates that some of the particles have a pore on their surface. The average diameter of hollow sphere is still 150–200 nm.Fig. 2 shows the typical XRD pattern of the as-prepared MSH, MSP and SSH sub-micron spheres. All the XRD patterns contain five distinguishable and broadened diffraction peaks at 29.63°, 36.50°, 42.40°, 61.52°, 73.70°, 77.57°, respectively. The peaks can be perfectly indexed to (110), (111), (200), (220), (311) and (222) lattice planes of cubic Cu2O (JCPDS file no. 65-3288). The XRD pattern of MSP and SSH spheres also contain other weak CuO peaks at 35.50°, 38.73°, 48.73°, respectively. i.e. (002), (111), (202) lattice planes of monoclinic CuO (JCPDS file no. 48-1548) which means CuO was formed with the increase of reaction time. But the main component of MSPS and SSHS is still Cu2O.
3.2. Formation mechanism of multi-shelled Cu2O spheres
Some researchers studied the formation mechanism of multi-shelled Cu2O spheres. Xu et al.29 pointed out that CTAB plays a vital template-mediated role in the formation of different Cu2O hollow architecture. However, Qi et al.31 found that Xu's method is hard to extend to other materials due to the unstable structure of vesicle templates and their high sensitivity on reaction environments. In previous researches, VC usually was thought as reducing agent. We found that except CTAB, VC also works as a part of template in the synthesis of Cu2O hollow structure. Three experiments were carried out to testify our assumption. The results are shown in Fig. 3. If we use glucose with the same mole number as a reducing agent, after adding NaOH solution, the color of the solution changed from blue to white-blue. After three minutes of reaction, the solution turns to be yellow. The reaction product is solid Cu2O octahedron rather than sphere, as shown in Fig. 3(a). If we used hydrazine with the same mole number as a reducing agent, the color of the solution turned from blue to yellow immediately after we added the hydrazine into Cu2SO4 solutions. In this reaction, solid Cu2O spheres with some small particles attached on them were formed, as shown in Fig. 3(b). The reducibility of VC is stronger than glucose but weaker than hydrazine. None of hollow structure was obtained by other two reducing agents. Meanwhile, if we change the adding order of VC and NaOH in the experiment. It could be found that Cu2O dodecahedron rather than sphere was obtained, as shown in Fig. 3(c). So we conjecture that except working as reducing agent, VC also works as a supplementary template in the formation of different Cu2O hollow spheres. | ||
| Fig. 3 SEM images of products obtained by different chemical reactions. (a) Using glucose as the reductant, (b) using hydrazine as the reductant, (c) change the adding order of VC and NaOH. | ||
The formation mechanism of Cu2O hollow structure is shown in Fig. 4. In the initial stages of the reaction, CTAB is easy to form micelles and closed bilayer aggregates such as vesicles under the assist of VC, as shown in step I of Fig. 4. When CuSO4 solution is injected into the solution Cu2+ ions will be absorbs on the surface of CTAB vesicles. After a while, Cu2+ ions was uniformly distributed in the vesicle shell, as shown in step II of Fig. 4. When NaOH was added in the solution, multi-shelled Cu(OH)2 spheres were formed immediately and be simultaneously reduced into Cu2O by VC, as shown in step III of Fig. 4. The formation process of multi-shelled hollow Cu2O spheres can be described by reaction eqn (1) and (2).
| Cu2+ + 2OH− → Cu(OH)2↓ | (1) |
| 2Cu(OH)2 + C6H8O6 → Cu2O↓ + C6H6O6 + 3H2O | (2) |
From above experiments, we can find that CTAB vesicles are unstable in alkaline solution. Using glucose as a reducing agent, reducing rate of Cu(OH)2 is slowly. Moreover, the CTAB vesicle is damaged in the alkaline solution. When the Cu2O nano particles are formed in the solution, they tend to attach on the existing Cu2O particles and forms Cu2O octahedrons by orientation growth. When we change the adding order of VC and NaOH, CTAB vesicles are destroyed firstly. Similarly, Cu2O tend to orientation growth, we just obtain Cu2O dodecahedrons. Hydrazine is an alkaline reducing agent and the reducing rate of hydrazine is much faster than glucose and VC, when the hydrazine is adding into CuSO4 solution, lots of Cu2O nano particles are formed at the same time. These particles will aggregate and forms solid Cu2O spheres. During the course, CTAB vesicles are destroyed, because we do not find any hollow structure in the product.
3.3. Microstructure evolution of Cu2O spheres in alkali solutions
On the basis of the above analysis, the formation process and mechanism of multi-shelled Cu2O hollow structures could be illustrated by Fig. 4. But the structure evolution of Cu2O hollow structures is not well understood.A time-dependent experiment was carried out to investigate the structural evolution of Cu2O hollow structure. Using the typical synthesis procedure, the samples obtained at various reaction times were inspected by TEM, as shown in Fig. 5(a)–(f). We found that MSH sub-micron spheres can be synthesized after five seconds of reaction (Fig. 5(a)). After 3 minutes of reaction, MSH and MSP sub-micron spheres can be observed in the product (Fig. 5(c)). When we prolong the reaction time to 10 min, MSH sub-micron spheres completely transformed into MSP sub-micron spheres. If the reaction time was further increased to 30 minutes, some SSH sub-micron spheres were observed in the product (Fig. 5(e)). After 60 minutes' reaction, all the products are SSH sub-micron spheres, as shown in Fig. 5(f). The structural evolution of Cu2O hollow structures is illustrated in step III of Fig. 4. Though the average size of Cu2O hollow structure changes little, we can find that the shell thickness of the particles increases with the increase of reaction time. The reaction equation of the Cu2O in alkaline solution could be described as follows:32
| Cu2O + OH− + H2O → [Cu(OH)2]− + CuOH | (3) |
| 2CuOH → Cu2O + H2O | (4) |
| 4CuOH + O2 → 4CuO + 2H2O | (5) |
 | ||
| Fig. 5 TEM images of Cu2O hollow structures obtained at various reaction times: (a) 5 s, (b) 30 s, (c) 3 min, (d) 10 min, (e) 30 min, (f) 1 h. | ||
In alkaline solution, Cu2O could reaction with OH− and forms [Cu(OH)2]− and CuOH. [Cu(OH)2]− dissolves in water, CuOH is not stable, it's easy to decompose and forms Cu2O or CuO (when O2 exist). In this course, the pH value of the solution decrease gradually, which is consistent with our measurement of the pH in the solution, as shown in Fig. S2 (see ESI†). Moreover, when OH− etch the surface and interior of Cu2O MSH sub-micron spheres, Cu2O continue to dissolve, which will form holes on the MSH sub-micron spheres and result in MSP sub-micron spheres. Due to the dissolution and precipitation of Cu2O, Ostwald ripening happened. The new generated Cu2O would deposit on the interior surface to increase the thickness of the shell. The formation process of MSHS can be described by reaction eqn (3) and (4). Since the inner smaller particles have a higher specific surface area, so the dissolution rate of inner particles is relatively fast than the external surface. Ostwald ripening and surface reconstruction will make multi-shelled hollow structure transform into single-shelled hollow structures in the following 1 hour. Meanwhile, because there is some O2 in the solution, reaction eqn (5) would happen and CuO will form. These phenomenon can be verified by the XRD pattern (Fig. 2). If we increase the mole number of NaOH to 2.5 M, the Cu2O multi-shelled hollow structure will transform into single-shelled hollow structures in 10 minutes, as Fig. S3 shows (see ESI†).
3.4. Relationship between microstructure and photoelectrochemical response
Fig. 6 is the photoelectrochemical response of Cu2O different hollow micro-/nanostructures in 0.5 M Na2SO4 solution under 300 W Xe lamp (intensity: 100 mW cm−2) with 5 s light on/off cycles through AM 1.5G optical filter illumination. This photo-induced cathodic current results from the reduction of protons involving the photo-generation of electrons, and reveals the p-type nature of the Cu2O photoelectrode.33 It also reveal the interfacial generation and separation dynamics of photogenerated charges of semiconductor photocatalysts. A larger photocurrent indicates higher electrons and holes separation efficiency.34 The results indicate that three Cu2O hollow spheres obtained in this paper have strong response to the sun light. The photocurrent values of MSH, MSP and SSH sub-micron sphere films at −0.45 V versus Ag/AgCl are about 0.08 mA cm−2, 0.06 mA cm−2, 0.07 mA cm−2, respectively. The MSH sub-micron spheres have highest photocurrent than the other two materials. | ||
| Fig. 6 Current density–potential responses of different Cu2O hollow micro-/nanostructures in 0.5 M Na2SO4 solution under 300 W Xe lamp through AM 1.5 optical filter illumination. | ||
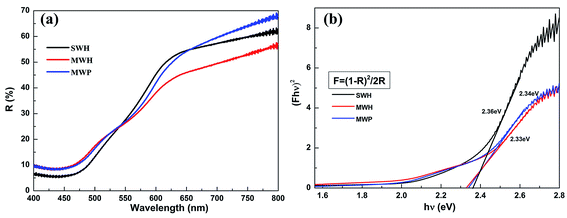
The photocurrent values indicate that MSH sub-micron spheres has the best photoelectrochemical response, but the reason is not clear. The ultraviolet-visible reflectance spectra experiment was carried out to study the corresponding band gaps of three samples.
Fig. 7(a) shows the ultraviolet-visible diffuse reflectance spectra of three samples. The corresponding band gaps were obtained from the corresponding modified Kubelka–Munk function,35 as shown in Fig. 7(b). The band gap of MSH, MSP and SSH sub-micron spheres are 2.33 eV, 2.36 eV, and 2.34 eV, respectively. It can be seen that MSH sub-micron spheres have a slightly lower band gap than SSH and MSP sub-micron spheres, which is in agreement with its lower reflectance in Fig. 7(a). That is to say, MSH sub-micron spheres have larger absorption range and could harvest more solar energy to generate more electron–hole pairs to join in the redox reaction on the surface, which will produce bigger photocurrent.
4. Conclusion
In summary, in this paper Cu2O single-shelled hollow spheres, multi-shelled hollow spheres and multi-shelled porous spheres were successfully synthesized via a facile and one-pot route in the presence of hexadecyltrimethylammonium bromide (CTAB) and ascorbic acid (VC). The morphology and structure of Cu2O nanoparticles can be adjusted by changing alkalinity and duration. CTAB and VC work as a template-mediated and OH− work as an etching agent in the formation of different hollow structures. The obtained different morphologies Cu2O hollow spheres were active photocatalysts for solar water-splitting. The results indicate that these special morphologies Cu2O hollow spheres have strong response under the simulated sunlight (the AM 1.5 spectrum) illumination. The Cu2O multi-shelled hollow spheres demonstrated an enhanced photocatalytic. Further investigation indicates that the difference of PEC efficiency should attribute to the Cu2O MSH sub-micron spheres have smaller band gap and larger light absorption.Acknowledgements
This work is supported by the National Nature Science Foundation of China (11575025) and the Fundamental Research Funds for the Central Universities.References
- A. D. Dinsmore, M. F. Hsu, M. G. Nikolaides, M. Marquez, A. R. Bausch and D. A. Weitz, Science, 2002, 298, 1006 CrossRef CAS PubMed
.
- Y. G. Sun and Y. N. Xia, Science, 2002, 298, 2176 CrossRef CAS PubMed
.
- Y. Yin, R. M. Rioux, C. K. Erdonmez, S. Hughes, G. A. Somorjai and A. P. Alivisatos, Science, 2004, 304, 711 CrossRef CAS PubMed
.
- H. G. Yang and H. C. Zeng, Angew. Chem., Int. Ed., 2004, 43, 5930 CrossRef CAS PubMed
.
- J. F. Chen, H. M. Ding, J. X. Wang and L. Shao, Biomaterials, 2004, 25, 723 CrossRef CAS PubMed
.
- J. Yang, L. Qi, C. Lu, J. Ma and H. Cheng, Angew. Chem., Int. Ed., 2005, 44, 598 CrossRef CAS PubMed
.
- J. Y. Chen, F. Saeki, B. J. Wiley, H. Cang, M. J. Cobb, Z. Y. Li, L. Au, H. Zhang, M. B. Kimmey, X. D. Li and Y. N. Xia, Nano Lett., 2005, 5, 473 CrossRef CAS PubMed
.
- X. W. Lou and L. A. Archer, Adv. Mater., 2008, 20, 1853 CrossRef CAS
.
- Q. H. Cui, Y. S. Zhao and J. N. Yao, J. Mater. Chem., 2012, 22, 4136 RSC
.
- N. Venugopal, D. J. Lee, Y. J. Lee and Y. K. Sun, J. Mater. Chem. A, 2013, 1, 13164 CAS
.
- K. J. C. van Bommel, A. Friggeri and S. Shinkai, Angew. Chem., Int. Ed., 2003, 42, 980 CrossRef CAS PubMed
.
- X. W. Lou, L. A. Archer and Z. Yang, Adv. Mater., 2008, 20, 3987 CrossRef CAS
.
- H. G. Yang and H. C. Zeng, Angew. Chem., Int. Ed., 2004, 43, 5206 CrossRef CAS PubMed
.
- H. C. Zeng, J. Mater. Chem. A, 2011, 21, 7511 RSC
.
- Y. Sun, B. Mayers and Y. Xia, Adv. Mater., 2003, 15, 641 CrossRef CAS
.
- K. An and T. Hyeon, Nano Today, 2009, 4, 359 CrossRef CAS
.
- Q. Zhang, T. Zhang, J. Ge and Y. Yin, Nano Lett., 2008, 8, 2867 CrossRef CAS PubMed
.
- K. Y. Niu, J. Yang, S. A. Kulinich, J. Sun, H. Li and X. W. Du, J. Am. Chem. Soc., 2010, 132, 9814 CrossRef CAS PubMed
.
- X. W. Lou, C. Yuan, Q. Zhang and L. A. Archer, Angew. Chem., Int. Ed., 2006, 45, 3825 CrossRef CAS PubMed
.
- M. R. Kim and D. J. Jang, Chem. Commun., 2008, 41, 5218 RSC
.
- H. L. Xu, W. Z. Wang and W. Zhu, J. Phys. Chem. B, 2006, 110, 13829 CrossRef CAS PubMed
.
- H. G. Zhang, Q. S. Zhu, Y. Zhang, Y. Wang, L. Zhao and B. Yu, Adv. Funct. Mater., 2007, 17, 2766 CrossRef CAS
.
- X. Li, H. Gao, C. J. Murphy and L. Gou, Nano Lett., 2004, 4, 1903 CrossRef CAS
.
- M. Diab, B. Moshofsky, I. J. Plante and T. Mokari, J. Mater. Chem., 2011, 21, 11626 RSC
.
- W. Z. Wang, X. W. Huang, S. Wu, Y. X. Zhou, L. J. Wang, H. L. Shi, Y. J. Liang and B. Zou, Appl. Catal., B, 2013, 134, 293 CrossRef
.
- Y. Yao, M. T. McDowell, I. Ryu, H. Wu, N. Liu, L. B. Hu, W. D. Nix and Y. Cui, Nano Lett., 2011, 11, 2949 CrossRef CAS PubMed
.
- C. H. Lu, L. M. Qi, J. H. Yang, X. Y. Wang, D. Y. Zhang, J. L. Xie and J. M. Ma, Adv. Mater., 2005, 17, 2562 CrossRef CAS
.
- Y. Chang, J. J. Teo and H. C. Zeng, Langmuir, 2005, 21, 1074 CrossRef CAS PubMed
.
- H. L. Xu and W. Z. Wang, Angew. Chem., 2007, 46, 1489 CrossRef CAS PubMed
.
- J. Y. Wang, H. J. Tang, L. J. Zhang, H. Ren, R. B. Yu, Q. Jin, J. Qi, D. Mao, M. Yang, Y. Wang, P. R. Liu, Y. Zhang, Y. R. Wen, L. Gu, G. H. Ma, Z. G. Su, Z. Y. Tang, H. J. Zhao and D. Wang, Nature Energy, 2016, 1, 1 Search PubMed
.
- J. Qi, X. Y. Lai, J. Y. Wang, H. J. Tang, H. Ren, Y. Yang, Q. Jin, L. J. Zhang, R. B. Yu, G. H. Ma, Z. G. Su, H. J. Zhao and D. Wang, Chem. Soc. Rev., 2015, 44, 6749 RSC
.
- A. A. Aref, L. B. Xiong, N. N. Yan, A. M. Abdulkarem and Y. Yu, Mater. Chem. Phys., 2011, 127, 433 CrossRef CAS
.
- Y. K. Hsua, C. H. Yua, Y. C. Chen and Y. G. Lin, Electrochim. Acta, 2013, 105, 62 CrossRef
.
- Y. M. He, J. Cai, T. T. Li, Y. Wu, H. J. Lin, L. H. Zhao and M. F. Luo, Chem. Eng. J., 2013, 215, 721 CrossRef
.
- T. J. McCarthy, T. A. Tamer and M. G. Kanatzidis, J. Am. Chem. Soc., 1995, 117, 1294 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra22474a |
| This journal is © The Royal Society of Chemistry 2016 |