Evaluation of physical structural features on influencing enzymatic hydrolysis efficiency of micronized wood
Abstract
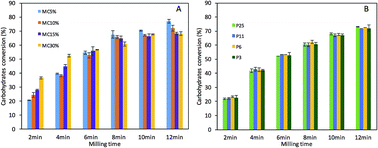
Enzymatic hydrolysis of lignocellulosic biomass is highly dependent on the changes in structural features after pretreatment. Mechanical milling pretreatment is an effective approach to alter the physical structure of biomass and thus improve enzymatic hydrolysis. This study examined the influence of structural characteristics on the enzymatic hydrolysis of micronized wood particles from mechanical milling pretreatment. We have also evaluated the energy efficiency of this processing method. Results indicate that the influence of processing variables on enzymatic hydrolysis of micronized wood relate mainly to the structural properties of particles. Reducing particle size down to ca. 30 μm disintegrates fibers and fiber bundles, while improving the enzymatic hydrolysis of the milled wood to around 40% of theoretical yield. Mechanically disintegrating the fiber cell wall into micronized fragments smaller than 30 μm further increases surface area and disrupts crystalline structure of cellulose, facilitating significant carbohydrate conversion (over 70% of theoretical yield). Empirical prediction of carbohydrate conversion with structural characteristics using a multiple linear regression model indicated that the enzymatic hydrolysis of micronized wood improved as collectively increasing surface area (i.e., reducing particle size and aspect ratio) and decreasing crystallinity during mechanical milling pretreatment. Energy efficiency results demonstrate that using a low-moisture content of the starting material and a multi-step milling process decreases the energy required when producing simple sugars with a mechanical pretreatment. Findings from this study provide new insights for mechanically overcoming biomass recalcitrance and developing cost-effective milling technologies for industrial scale applications in biorefinery.

 Please wait while we load your content...
Please wait while we load your content...