Theoretical study on the mechanism of the gas phase reaction of methoxybenzene with ozone†
Jianfei Sun,
Haijie Cao,
Shiqing Zhang,
Xin Li and
Maoxia He*
Environment Research Institute, Shandong University, Jinan 250100, P. R. China. E-mail: hemaox@sdu.edu.cn
First published on 29th November 2016
Abstract
Methoxybenzene (MB), is seen as a potential air pollutant which may cause environmental issues in the troposphere. Nevertheless, our understanding of its gas phase reaction is extraordinarily limited. In this study, the quantum chemical methods [M06-2X/aug-cc-pVDZ//M06-2X/6-31+g(d,p)] and Rice–Ramsperger–Kassel–Marcus (RRKM) theory are utilized in investigating the detailed mechanisms and rate constants for the ozonolysis of MB for the first time. The results show that O3-addition to the methoxy-substituted carbon dominates the entrance channel of MB with ozone and the total rate coefficient of the title reactions is calculated to be 2.67 × 10−21 cm3 per molecule per s at 298 K and 1 atm. The bimolecular rate constants show positive dependence on temperature (200–400 K) and negative dependence on pressure (10−3, 10−2, 10−1 and 1 atm). Moreover, the atmospheric lifetime of MB determined by O3 is available concerning the typical concentration of O3. This work aims to provide reference data for future experimental research on MB.
1. Introduction
Lignin as one of the basic elements of natural wood, is the most abundant substance in nature composed of aromatic moieties.1–3 Huge amounts of lignin are produced by the paper industries every year. Lignin is widespread in our daily life. The thermal decomposition of biomass lignin can generate high concentrations of phenolic molecules.4 In this regard, anisole (methoxybenzene, MB) as a phenolic compound is released to the atmosphere from biomass burning. Previous research has shown that biomass fuels can increase the risk of lung cancer.5 As a result, MB is an air pollutant consisted in the wood smoke and endangers human health.MB serves for many purposes, it not only can be made into a solvent, but also widely used in the preparation of many floral essences.6 In addition, MB acts as an important intermediate of organic synthesis. MB belongs to the volatile organic compounds (VOCs), because the saturated vapor pressure of MB is 470 Pa at room temperature, and its boiling point is 153.8 °C.7 VOCs in the troposphere are mainly derived from anthropogenic and natural sources.8,9 With the development of science and technology, the types of VOCs are really quite abundant, while most of them are poisonous and harmful substance.10,11 Many preparation methods of MB are discovered, therefore the use of MB also increases. Once entered or produced in the atmosphere, MB will proceed to react with oxidants in the air, such as OH radicals, nitrate radicals, Cl atoms and ozone.
In order to make a thorough research, the reaction of MB with OH radicals, Cl atoms and ozone should be studied clearly. Perry et al. and Ce'cile Coeur-Tourneur et al. have determined the rate constants for the reaction of hydroxyl radicals with MB respectively.12,13 Recently, the rate coefficients of the reactions of NO3 and Cl atoms with MB have been studied respectively by Amélie Lauraguais et al.14,15 These findings are important for future research. Furthermore, as known to all, ozone is a significant reactive oxidant in the troposphere, which plays an important role in the removal of air pollutants. Consequently, the reaction mechanism and kinetic information on ozonolysis of MB are essential to assess the possible effect of MB to air pollution in the troposphere. Nevertheless, to the best of our knowledge, there is no available experimental and theoretical study about the gas phase reaction of MB and ozone. However, guaiacol, which has a similar structure to MB, is chosen as a reference compound to understand the property of MB.
Then on this basis, in order to deepen the understanding of the gas phase reaction of MB with ozone, we perform a systematic theoretical calculation for this reaction by means of quantum chemical approaches in the framework of density functional theory (DFT).16–18 The complete mechanisms include the generation and decomposition of primary ozonides (POZs) formed from MB + O3, as well as the further reactions of Criegee intermediates in the presence of NO/H2O. Moreover, the rate constants of ozonolysis of MB are investigated as functions of temperatures and pressures and the O3-determined atmospheric lifetime of MB is also estimated.
2. Computational details
2.1 Electronic structure calculations
In order to find out an accurate and efficiency method, several functions with and without dispersion corrections were applied to determine the energy parameters of a representative addition reaction. The representative reaction of MB + O3 → primary ozonide (IM1) was investigated to estimate the reliability of theoretical methods used in the Table S1 (in ESI†). The energies calculated at the [CCSD(T)/6-31+g(d,p)//M06-2X/6-31+g(d,p)] level were employed as benchmark data to access the reliability of different methods.19 Among these methods, geometries and energies at M06-2X level are similar to the results of CCSD(T). Hence, M06-2X is appropriate in calculating the parameters of ozonolysis of MB considering both accuracy and efficiency. The geometries of all the stagnation points (reactants, products, intermediates) and saddle points (transition states) are optimized at the M06-2X/6-31+g(d,p) level. The M06-2X functional is an efficient method of computation that has been proved to be especially exact for the determination of thermodynamics for systems including main-group elements.20 This method was proven be reliable and appropriate (accurate and time-saving) in calculations as discussed above. Based on optimized configuration, the frequency analysis were carried out to ensure that no imaginary frequency existed in stationary point and every saddle point had one and only one imaginary frequency at the same level. Intrinsic reaction coordinates (IRC) calculations21,22 were performed to verify that transition structures connected to their corresponding expected reactants and products. The GAUSSIAN 09 package23 was applied to carry out all the structure and energy calculations reported in this paper. Although CCSD(T) is regarded as a highly accurate calculation method,24 it will cost a lot of time with high size basis set for MB + O3. In order to get more accurate and effective energy, we used M06-2X functional with four different basis sets aug-cc-pVDZ,25 aug-cc-pVQZ, aug-cc-pVTZ and 6-311++g (3df,2p) to calculate the single-point energies as shown in Table S1.† By comparison, the aug-cc-pVDZ basis set was suitable to calculate reliable data and adopted to the single point-energy calculations of all optimized structures in the same M06-2X level in this paper.2.2 Rate constant calculations
Based on the results of thermodynamic parameters, rate constants of elementary reactions were obtained by solving the energy-grained master-equation on the MultiWell software26,27 using Rice–Ramsperger–Kassel–Marcus (RRKM) theory.28–30 It is routinely applied to compute the kinetics of air pollution.31 Argon was selected as the collider bath gas with Lennard-Jones parameters of σ = 3.47 Å, ε/kb = 114 K.32 The densities of states of reactants, intermediates and active compounds were computed with energy grain size of 10 cm−1. The detailed formula of the energy-dependent specific rate constant and other parameters (the collision model, Lennard-Jones of intermediates, etc.) in performing kinetic calculations are provided in ESI.†3. Results and discussion
3.1 Mechanism of reactions
Owing to the internal rotation of C7–O1 single bond, MB has two kinds of stable structures. By comparing the zero-point corrected energies of the structures, structure 1 as shown in Fig. 1 has lower energy and is selected to perform the following calculations in this paper. This is in agreement with the results given by H. M. Seip.33The detailed reaction mechanisms of MB + O3 reaction mentioned in this paper are displayed in Fig. 2(a–c). Others are shown in Fig. S1 (in ESI†). In order to understand the reaction mechanism better, gives the potential energy surfaces (PESs) with zero-point correction for the initial reaction of MB + O3. The optimized geometrical structures of transition states, involved in the first step of initial reaction, with main bond lengths, are shown in Fig. 4. And the optimized geometrical structures of the Criegee intermediates are provided in Fig. S2.†
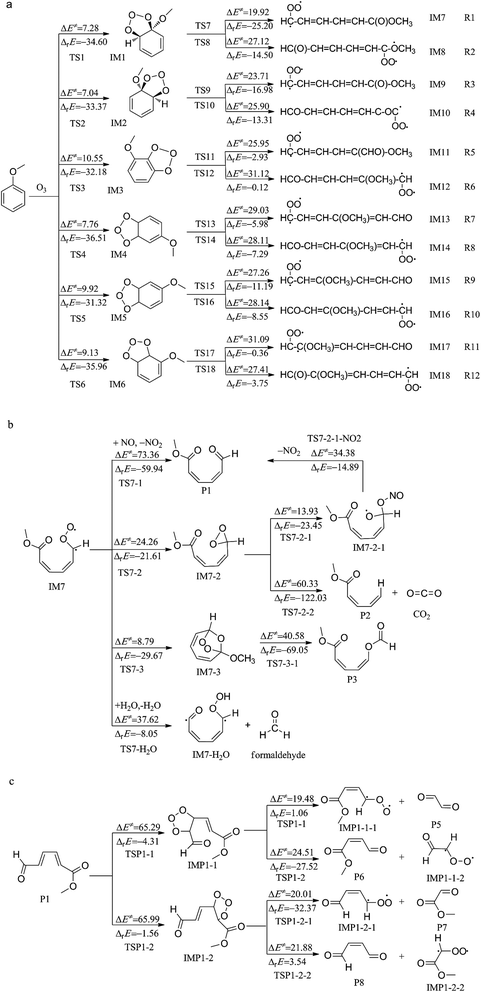 | ||
| Fig. 2 The detailed mechanisms of MB with ozone: (a) is the initial reaction, (b) (c) are the further reactions of IM7 and P1, respectively. | ||
The ozonolysis of MB initiates with two terminal O atoms of O3 molecule simultaneously adding to adjacent C atoms of phenyl ring of MB, and then the primary ozonide (POZ) is formed. Fig. 2a has shown the detailed reaction parameters for the six entrance channels.
O3 molecule adding to C1 and C6 atoms of benzene ring will form primary ozonide IM1 via the transition state TS1. As shown in Fig. 4, O3 cycloaddition results in the formation of a five-membered ring. The transition state structures were verified using frequency calculations. In IM1, the bond lengths of C1–O2, C6–O4 respectively are 1.446 and 1.430 Å, which are shortened by 35.21 and 27.07% in TS1, respectively. The energy barrier and exothermic heat of this process are 7.28 and 34.60 kcal mol−1, respectively. With respect to the para-position of C1 and C6, ozone reacting with C3, C4 leads to the generation of POZ IM4, in which the distance between C3–O2 and C4–O4 are 1.440 and 1.434 Å, respectively. This process suffers the energy barrier of 7.75 kcal mol−1 via TS4 and is exothermic by 36.51 kcal mol−1. TS2 and TS5 are the transition states formed by O3 attacking two pairs of adjacent carbon atoms (C1 and C2, C4 and C5) with the energy barriers of 7.04 and 9.92 kcal mol−1, respectively. IM2 and IM5 are the corresponding addition products with 33.37 and 31.32 kcal mol−1 higher in energy than the reactants. Another reaction of the formation of transition states (TS3, TS6) and primary ozonides (IM3, IM6) follow the same trends as mentioned above. The two outer O atoms of the O3 group are oriented towards the corresponding C–C bond in all cases. The calculated values of IM3 and IM6 are 32.18 and 35.96 kcal mol−1 lower than that of the reactants. It means that both reactions are exothermic like the primary reactions we described before.
To conclude, all the pathways of the O3-addition reaction can occur due to the high exothermicity and moderate energy barriers. Among these reactions, the formation of IM1 and IM2 are much more favorable than POZs. These calculated outcomes can fit well with the following kinetic calculation.
The POZs are not stable in the atmosphere. As shown in Fig. 2a, the O3-addition product, IM1, is an energy-abundant compound which will undergo the decomposition to form the Criegee intermediates IM7 and IM8 via transition states TS7 and TS8, respectively. The cleavages of O2–O3 and O3–O4 bonds in the primary ozonides lead to the formation of different intermediates. In TS7, O2–O3 and C1–C6 bonds tend to break up simultaneously resulting in the destruction of six-member-ring structure of benzene. Besides, the original C1–O2 single bond changes into C1–O2 double bond. The bond length of C1–O2 in TS7 is 1.279 Å which is 0.07 Å longer than that in IM7. IRC calculation shows that TS7 does connect IM1 and IM7. Additionally, IM7 is produced through TS7 with the energy barrier of 19.92 kcal mol−1 and 25.20 kcal mol−1 exothermic value is released for further reaction. Similarly, the generation of IM8 is accompanied by the cleavages of O3–O4 and C1–C6 bonds and the formation of C4–O6 double bond via TS8. In summary, all the POZs formed in the entrance channels are prior to undergo intermolecular decomposition and produce two Criegee intermediates by similar reaction mechanism as mentioned above (Fig. 3).
 | ||
| Fig. 3 Profiles of the energy surface for the initial reaction of MB + O3, and the followed self-decomposition. | ||
 | ||
| Fig. 4 M06-2X/6-31+g(d,p) optimized geometries for the transition states involved in the reaction of MB + O3 with main bond length. Bond lengths are in Å. | ||
Criegee intermediates are known to be reactive and easily undergo further reactions in the atmosphere. In the following section, IM7 and IM8 are selected as represents to discuss their properties completely. The further reactions of other Criegee intermediates are shown in ESI (Fig. S1†), and will not be described in detail here.
3.2 Secondary reactions
The Criegee intermediates generated in the first reactions can dissociate or react with NO and H2O which abundant in atmosphere respectively.IM7 has two single electrons at O3 and C6 atoms, respectively and is inclined to undergo subsequent reactions. In TS7-2, IM7 undergoes self-decomposition via O3 atom associating with C6 atom which forms a three-membered ring structure. The bond lengths of C6–O4, C6–O3 and O3–O4 are 1.390, 1.390 and 1.471 Å in the intermediate IM7-2, respectively. This process needs to go through the energy barrier by 24.26 kcal mol−1 via TS7-2 and is exothermic by 21.61 kcal mol−1.
Fig. 2b has shown two channels for further reaction of IM7-2. First, NO radical can directly abstract the terminal O atom of IM7-2 to form IM7-2-1 via transition state TS7-2-1 with the energy barrier of 13.93 kcal mol−1. The three-numbered ring is destroyed as a result of the cleavage of O3–O4 bond and the formation of N1–O3 bond. TS7-2-1 is the transition state in which the bond lengths of N1–O3 and C6–O3 are 1.964 (1.395 Å in IM7-2-1) and 1.398 Å (1.432 Å in IM7-2-1). IM7-2-1 is not stable which will be followed by the NO abstraction O atom via transition state TS7-2-1-NO2 and finally leads to the formation of P1 + NO2. This process is not favorable due to the high energy barrier of 34.38 kcal mol−1. The second self-decomposition of IM7-2 is carried out by the rupture of C6–O4 and C6–H5 bonds and the H5 attached to C6 is transferred to C5. The generation of (Z)-methyl penta-2,4-dienoate (P2) and CO2 is an endothermic process with the liberated heat of 122.03 kcal mol−1 and energy barrier of 60.33 kcal mol−1. The high energy barrier and high exothermicity indicate that the channel towards P2 reaction can occur in high temperature.
With regard to the third reaction channel of IM7, a seven-member-ring structure is generated in virtue of the formation of C6–O2 and C1–O3 bonds. The bond lengths of C6–O2 and C1–O3 in TS7-3 are elongated by 42.89 and 45.13% with respect to that in IM7-3, respectively. The reaction of the generation of IM7-3 is exothermic by 29.67 kcal mol−1, while the computed energy barrier is 8.79 kcal mol−1. This intermediate can continue to react. Newly formed seven-member-ring structure is destroyed via TS7-3-1. This process is the destruction of seven-numbered ring structure with the transition state of TS7-3-1, resulting in (2Z,4Z)-methyl-5-(formyloxy)-penta-2,4-dienoate (P3). This is also an endothermic pathway by releasing 69.05 kcal mol−1 heat quantities, with the energy barrier of 40.58 kcal mol−1.
Since Criegee intermediate are the crucial species formed from the ozonolysis of MB. Besides, IM7 in the atmosphere can be removed through bimolecular reactions. IM7 can react with H2O in the troposphere. Owing to the participation of H2O, H atom of the methoxyl group is transferred to the OH group in H2O and meanwhile H atom in H2O is being attached to O3 atom producing new hydroxyl. The water molecules play the role of ‘water bridge’ essentially. This process results in the formation of IM7–H2O and formaldehyde via the liberated heat of 8.05 kcal mol−1 and energy barrier of 37.62 kcal mol−1.
Through above analysis we can see that there are two formation channels of (2Z,4Z)-methyl-6-oxohexa-2,4-dienoate (P1) which are both NO-abstraction processes. That is to say, the main product P1 is formed after the oxygen atom abstraction by NO.
First, NO possesses free radical, which makes it can react with IM8 easily. The reaction is essentially a process of O-abstraction in IM8 via transition state TS8-1. The bond length of O2–O3 in TS8-1 is elongated by 17.69% compared to IM8. The reason for this situation is O3 atom is abstracted by NO. In this process, P1 and NO are produced by overcoming 8.62 kcal mol−1 energy barriers, and releasing 70.64 kcal mol−1 exothermic heats. The low energy barrier and high exothermicity make the abstraction reaction can occur easily. IRC results show an obvious oxygen transformation from IM8 to NO radical. What's more, IM7 and IM8 both produce P1 through reactions with NO radical. The further reaction mechanism of P1 will be introduced in next chapter.
With respect to the second reaction channels of IM8, O3 atom migrates from O2 to C1 forming an intermediate (IM8-2) which contains a three-membered ring composed of C1, O2 and O3. The O2–O3 bond is stretched to 1.489 Å approximately, and the newly formed C1–O3 bond is about 1.387 Å, meanwhile the bond length of C1–O2 is elongated by 8.11%. The barrier height for TS8-2 is about 13.12 kcal mol−1. And IM8-2 lies 21.85 kcal mol−1 below the reactant (IM8). Afterwards, for IM8-2, it can undergo further decomposition via reaction with NO or rearrangement. First, similar to the reaction of IM7-2 and NO, N atom attaches to O3, which results in the formation of IM8-2-1 via transition state TS8-2-1. This step is endothermic by 14.06 kcal mol−1 with an energy barrier of 11.68 kcal mol−1. Subsequently, the intermediate IM8-2-1 can undergo degradation through the rupture of O2–O3 bond, yielding the product P1 and NO2 radical. This process suffers a high energy barrier of 49.29 kcal mol−1, and hence is not favorable to occur. The other reaction is the rearrangement of IM8-2. In this process, the location of C1 is replaced by O3, leading to the generation of methyl ((1Z,3Z)-5-oxopenta-1,3-dien-1-yl) carbonate (P4). The product P4 lies 79.89 kcal mol−1 below IM8-2, while the transition state TS8-2-2 lies 83.52 kcal mol−1 above IM8-2. This path is not favorable to occur based on thermodynamic analysis.
The last reaction channel of IM8, the O3 atom will attach onto the C1 atom to form an intermediate IM8-3 with a seven-membered ring. This process is similar to the further reaction route of IM7.
In the ozonolysis reaction of (Z)-methyl 4-oxobut-2-enoate (P1), cycloaddition of ozone on the double bond is involved. IMP1-1 is the resulting ozonide containing a five-member-ring structure in which the distances between C and O are 1.417 (C2–O5) and 1.424 Å (C3–O7), respectively. This step is exothermic by 4.31 kcal mol−1 with a high energy barrier of 65.29 kcal mol−1. Due to the addition product IMP1-1 has two different O–O bonds, there are two kinds of corresponding decomposition paths. The formation of IMP1-1-1 and oxalaldehyde (P5) can result from the direct cleavage of the O5–O6 bond in IMP1-1. If the O6–O7 bond breaks, IMP1-1-1 and (Z)-methyl 4-oxobut-2-enoate (P6) will generate. Nevertheless, the energy barrier of the initial step of P1 + O3 is so high that the further reaction will not be considered anymore in this study.
From above mechanistic analysis of the further reaction, we can see that NO-abstraction processes have relatively low energy barriers. That is to say, these reaction routes are thermodynamically preferable. Additionally, the major products of the further reactions are (2Z,4Z)-methyl-6-oxohexa-2,4-dienoate (P1), (2Z,4Z)-methyl-5-(formyloxy)-penta-2,4-dienoate (P3), formaldehyde, and nitrogen dioxide. It is worth noting that the main products P1 and formaldehyde were also detected in the experiment for ozonation of MB in aqueous solution.34
3.3 Rate constant calculations
All rate constants in this work were calculated by solving the energy-grained master equation using RRKM theory on MultiWell-2014.1b program. Owing to RRKM theory is only applicable to unimolecular reactions systems, the reactants of bimolecular reactions (MB and O3) were considered as product set. For the initial step of the entrance channel of MB with O3 molecules, actually the high-pressure limiting rate constants of unimolecular decompositions (e.g. IM1 → MB + O3) k(inf)uni were obtained by MultiWell program. So taking advantage of equilibrium constant Keq, we can work out the high-pressure limit of the recombination (e.g. MB + O3 → IM1) rate constants k(inf)rec according to the eqn (1):| k(inf)rec = Keq × k(inf)uni | (1) |
| k(T,P) = k(inf)rec × f | (2) |
The energy-grained rate constant of elementary reaction can be obtained based on the sun and densities of states, vibrational frequencies, moment of inertia and formation of enthalpies of referred reactants, products and active compounds. The collision model detailed information is provided in the ESI.†
Based on the thermodynamic data, the overall and individual rate constants for the MB + O3 are calculated in the temperature range of 200–400 K at 1 atm, as listed in Table S2.† ki represents the individual rate constant of initial step Ri (e.g. MB + O3 → IM1).
Fig. 5 clearly shows the temperature dependence of the rate constants obtained for the six entrance channels at 1 atm. Within the studied temperature range (200–400 K), all of the calculated individual rate constants grow with increasing temperature. Therefore, the total reaction rate constant ktot is also positively correlated with temperature. In detail, ktot increases from 5.10 × 10−24 cm3 per molecule per s to 9.32 × 10−20 cm3 per molecule per s while the temperature ranges from 200 K to 400 K. The tot al reaction rate constant of MB + O3 is 2.67 × 10−21 cm3 per molecule per s under typical atmospheric temperature and atmospheric pressure. We did not find the pertinent literature with experimental data related to the rate constant of ozonolysis of MB, so it is difficult to compare our results with experimental data directly. Therefore we can make comparisons indirectly by estimation method. To be specific, guaiacol, which has a similar structure to MB, is chosen as a reference compound to understand the property of MB. The kinetics of guaiacol with O3 were carried out under dark conditions in a simulation chamber whose volume is 8 m3. The value kguaiacol = (4.0 ± 0.31) × 10−19 cm3 per molecule per s was determined by Atallah El Zein et al. at dry conditions, 294 ± 2 K, and atmospheric pressure.35 From this literature we can see that the rate constant for the reaction of guaiacol with O3 are more than 108 lower than those for reactions with OH radicals. Therefore, the order of magnitude of experimental rate constant for MB with O3 is 10−19 or 10−20 by estimation (the rate coefficient for the reaction of MB with OH radicals is 2.20 × 10−11 cm3 per molecule per s).13 From these observation we can conclude that the calculated rate coefficient of MB + O3 (2.67 × 10−21 cm3 per molecule per s) is relatively consistent with the estimated experimental result.
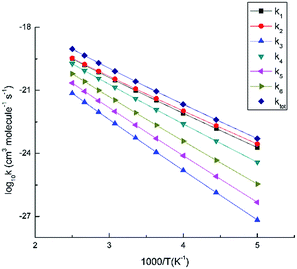 | ||
| Fig. 5 Calculated rate constants for the initial reaction of MB with O3 at different temperatures and 1 atm. | ||
In addition, from Fig. 5, it is clear that the values of k1 and k2 are significantly bigger than others throughout the studied temperatures. The values indicate that pathway 1 and pathway 2 are the favorable routes among all the O3-addition channels of MB in the temperature range of 200–400 K. In other words, O3 tends to attack on C1, C6 and C1, C2 two pairs of adjacent carbon atoms preferentially. This is in good agreement with the thermodynamic analysis.
To clarify the combined influence of the pressure and temperature on reaction, the rate constants of two reaction channels (MB + O3 → IM1 → IM7, MB + O3 → IM1 → IM8) have been calculated at several different pressures and temperatures. The corresponding calculated values are presented respectively in the form of table (Table 1) and figure (Fig. 6). From Fig. 6 we can see, the rate constants of the two channels are all show negative pressure dependence. The result suggests that these rate coefficients decrease with the increasing pressure. At the range of lower pressures and higher temperatures, the ozonolysis of MB are more likely to occur. Furthermore, it is worth noting that IM7 is the main Criegee intermediate from the decomposition of primary ozonide compared to IM8.
| ki | ||||||||
|---|---|---|---|---|---|---|---|---|
| Temperature T/K | MB + O3 → IM1 → IM7 | MB + O3 → IM1 → IM8 | ||||||
| 0.001 atm | 0.01 atm | 0.1 atm | 1 atm | 0.001 atm | 0.01 atm | 0.1 atm | 1 atm | |
| 200 | 1.874 × 10−24 | 1.499 × 10−24 | 3.358 × 10−24 | 3.861 × 10−26 | 1.484 × 10−26 | 1.139 × 10−26 | 1.796 × 10−27 | 2.363 × 10−28 |
| 225 | 1.493 × 10−23 | 1.295 × 10−23 | 3.506 × 10−24 | 4.217 × 10−25 | 1.306 × 10−25 | 1.001 × 10−25 | 2.447 × 10−26 | 2.259 × 10−27 |
| 250 | 8.026 × 10−23 | 7.384 × 10−23 | 2.438 × 10−23 | 3.014 × 10−24 | 8.191 × 10−25 | 6.183 × 10−25 | 2.068 × 10−25 | 3.041 × 10−26 |
| 275 | 3.259 × 10−22 | 3.109 × 10−22 | 1.262 × 10−22 | 1.744 × 10−23 | 3.458 × 10−24 | 2.726 × 10−24 | 1.103 × 10−24 | 1.318 × 10−25 |
| 298 | 9.731 × 10−22 | 9.477 × 10−22 | 4.604 × 10−22 | 7.046 × 10−23 | 1.16 × 10−23 | 9.926 × 10−24 | 4.16 × 10−24 | 5.908 × 10−25 |
| 325 | 2.940 × 10−21 | 2.906 × 10−21 | 1.688 × 10−21 | 2.973 × 10−22 | 4.144 × 10−23 | 3.459 × 10−23 | 1.819 × 10−23 | 3.205 × 10−24 |
| 350 | 7.128 × 10−21 | 7.094 × 10−21 | 9.192 × 10−23 | 9.655 × 10−22 | 1.097 × 10−22 | 9.192 × 10−23 | 5.953 × 10−23 | 1.393 × 10−23 |
| 375 | 1.562 × 10−20 | 1.555 × 10−20 | 2.454 × 10−22 | 2.785 × 10−21 | 2.411 × 10−22 | 2.454 × 10−22 | 1.848 × 10−22 | 4.758 × 10−23 |
| 400 | 3.133 × 10−20 | 3.131 × 10−20 | 5.444 × 10−22 | 7.351 × 10−21 | 5.645 × 10−22 | 5.444 × 10−22 | 3.811 × 10−22 | 1.228 × 10−22 |
 | ||
| Fig. 6 Calculated rate constants for the reaction of MB + O3 → IM1 → IM7 and MB + O3 → IM1 → IM8 at different temperatures and several pressures. | ||
3.4 Atmospheric implications
The atmospheric implications of MB emissions can be evaluated by comparing the lifetime of the reactions for MB with O3 molecules and other major oxidants in the troposphere. The results are presented in Table 2.| Reaction partner | Rate constant (cm3 per molecule per s) | Ref. | τa |
|---|---|---|---|
| a τ[OH] = 1 ÷ (k[OH]), where [OH] = 1.6 × 106 molecules per cm3. τ[NO3] = 1 ÷ (k[N3]), where [NO3] = 5 × 108 molecules per cm3. τ[Cl] = 1 ÷ (k[Cl]), where [Cl] = 5 × 104 atoms per cm3. τ[O3] = 1 ÷ (k[O3]), where [O3] = 7 × 1011 molecules per cm3. | |||
| OH | 2.20 × 10−11 | 13 | 7.9 h |
| NO3 | 1.07 × 10−10 | 14 | 52 h |
| Cl | 9.45 × 10−17 | 15 | 245 d |
| O3 | 2.64 × 10−21 | This work | 16.41 years |
For the bimolecular atmospheric reactions of MB + O3, the lifetime of the reactant τ is equal to the reciprocal of k[O3], which can evaluate the atmospheric implications of MB emissions. The equation is as follows:
| τ = 1 ÷ (k[O3]) | (3) |
From Table 2, we can see that the OH-determined lifetime of MB is 7.9 hours when the typical 12 h daily average OH radical concentration is 1.6 × 106 radicals per cm3. For the lifetime of MB with other oxidants, the corresponding atmospheric lifetime are 52 hours for NO3 radicals and 245 days for Cl atoms. Consequently, ozonolysis of MB is not important removal path and MB is difficult to degrade via ozonolysis in the atmosphere.
4. Conclusions
MB, a kind of potential air pollutant, can react with ozone in the atmosphere. By performing Density Functional Theory (DFT) calculations, a scientific theoretical study on the reaction mechanism and kinetics of MB + O3 were carried out. Some conclusions are drawn as follows:(1) In the reactions of ozone with MB, the mechanism are investigated for the first time: first, the formations of POZs occurred via O3 cycloaddition and two decomposition routes of POZs are followed subsequently. Among all the decomposition reactions of POZs, the formation channels of IM7 and IM8 are more favorable than others. This is in good agreement with the kinetic calculation. And the further reactions of Criegee intermediates (IM7 and IM8) proceed in the presence of H2O and NO.
(2) The main estimated products (2Z,4Z)-methyl-6-oxohexa-2,4-dienoate (P1), (2Z,4Z)-methyl-5-(formyloxy)-penta-2,4-dienoate (P3), formaldehyde, and nitrogen dioxide were obtained from calculation, of which P1 and formaldehyde were detected in the experiment for ozonation of MB in aqueous solution.
(3) At 298 K and 1 atm, the total rate constant of O3-initiated reactions of MB is 2.67 × 10−21 cm3 per molecule per s. Comparing with the previous experimental results of MB with OH radicals and reference compound (Guaiacol) with OH and O3 we concluded that our calculated results are reliable. This needs further identification by experimental studies. What's more, these rate constants are positively correlated with temperature (200–400 K). Additionally, the atmospheric lifetime of MB with respect to degradation by O3 is 16.41 years, indicating that MB is difficult to degrade in the atmosphere.
Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (NSFC No. 21377001, 21477065 and 21077067).References
- R. Rowell, The chemistry of solid wood, 1984 Search PubMed.
- R. C. Pettersen, The chemistry of solid wood, 1984, vol. 207, pp. 57–126 Search PubMed.
- F. G. Calvo-Flores and J. A. Dobado, ChemSusChem, 2010, 3, 1227–1235 CrossRef CAS PubMed.
- X. Zhu, L. L. Lobban, R. G. Mallinson and D. E. Resasco, J. Catal., 2011, 281, 21–29 CrossRef CAS.
- W. Y. Lim and A. Seow, Respirology, 2012, 17, 20–31 CrossRef PubMed.
- H. Fiege, H. W. Voges, T. Hamamoto, S. Umemura, T. Iwata, H. Miki, Y. Fujita, H. J. Buysch, D. Garbe and W. Paulus, Ullmann's Encyclopedia of Industrial Chemistry, 2000 Search PubMed.
- D. R. Lide and H. V. Kehiaian, CRC handbook of thermophysical and thermochemical data, Crc Press, 1994 Search PubMed.
- R. Atkinson, Atmos. Environ., 2000, 34, 2063–2101 CrossRef CAS.
- R. Atkinson and J. Arey, Chem. Rev., 2003, 103, 4605–4638 CrossRef CAS PubMed.
- S. Król, B. Zabiegała and J. Namieśnik, TrAC, Trends Anal. Chem., 2010, 29, 1092–1100 CrossRef.
- H. Singh, Y. Chen, A. Staudt, D. Jacob, D. Blake, B. Heikes and J. Snow, Nature, 2001, 410, 1078–1081 CrossRef CAS PubMed.
- R. Perry, R. Atkinson and J. Pitts Jr, J. Phys. Chem., 1977, 81, 1607–1611 CrossRef CAS.
- C. Coeur-Tourneur, A. Cassez and J. C. Wenger, J. Phys. Chem. A, 2010, 114, 11645–11650 CrossRef CAS PubMed.
- A. l. Lauraguais, I. Bejan, I. Barnes, P. Wiesen, C. c. Coeur-Tourneur and A. Cassez, J. Phys. Chem. A, 2014, 118, 1777–1784 CrossRef CAS PubMed.
- A. l. Lauraguais, A. El Zein, C. Coeur, E. Obeid, A. Cassez, M.-T. Rayez and J.-C. Rayez, J. Phys. Chem. A, 2016, 120, 2691–2699 CrossRef CAS PubMed.
- R. G. Parr, in Electron Distributions and the Chemical Bond, Springer, 1982, pp. 95–100 Search PubMed.
- R. G. Parr, in Horizons of Quantum Chemistry, Springer, 1980, pp. 5–15 Search PubMed.
- E. Runge and E. K. Gross, Phys. Rev. Lett., 1984, 52, 997 CrossRef CAS.
- B. Long, X. F. Tan, Y. B. Wang, J. Li, D. S. Ren and W. J. Zhang, ChemistrySelect, 2016, 1, 1421–1430 CrossRef CAS.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 CrossRef CAS.
- C. Gonzalez and H. B. Schlegel, J. Chem. Phys., 1989, 90, 2154–2161 CrossRef CAS.
- C. Gonzalez and H. B. Schlegel, J. Phys. Chem., 1990, 94, 5523–5527 CrossRef CAS.
- M. Frisch, G. Trucks, H. B. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, B. Mennucci and G. Petersson, Inc., Wallingford, CT, 2009, vol. 200.
- W. Klopper, J. Noga, H. Koch and T. Helgaker, Theor. Chem. Acc., 1997, 97, 164–176 CrossRef CAS.
- K. B. Wiberg, J. Comput. Chem., 2004, 25, 1342–1346 CrossRef CAS PubMed.
- J. Barker, et al., MultiWell Program Suite, version 2014.1 b, University of Michigan: Ann Arbor, MI, US, 2014 Search PubMed.
- J. R. Barker, Int. J. Chem. Kinet., 2001, 33, 232–245 CrossRef CAS.
- P. J. Robinson and K. A. Holbrook, Unimolecular reactions, Wiley-Interscience, New York, 1972 Search PubMed.
- W. Forst, Theory of unimolecular reactions, Elsevier, 2012 Search PubMed.
- R. G. Gilbert and S. C. Smith, Theory of unimolecular and recombination reactions, Blackwell Scientific Publications; Publishers' Business Services, 1990 Search PubMed.
- P. A. Ariya, R. Sander and P. J. Crutzen, J. Geophys. Res.: Atmos., 2000, 105, 17721–17738 CrossRef CAS.
- J. R. Barker, N. F. Ortiz, J. M. Preses, L. L. Lohr, A. Maranzana, P. J. Stimac, T. L. Nguyen and T. D. Kumar, MultiWell Program Suite, Ann Arbor, 2011, vol. 1001, pp. 48109–42143 Search PubMed.
- H. Seip and R. Seip, Acta Chem. Scand., 1973, 27, 4024–4027 CrossRef CAS.
- E. Mvula, S. Naumov and C. von Sonntag, Environ. Sci. Technol., 2009, 43, 6275–6282 CrossRef CAS PubMed.
- A. E. Zein, C. c. Coeur, E. Obeid, A. l. Lauraguais and T. Fagniez, J. Phys. Chem. A, 2015, 119, 6759–6765 CrossRef CAS PubMed.
- J. A. Logan, J. Geophys. Res.: Atmos., 1985, 90, 10463–10482 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra22286b |
| This journal is © The Royal Society of Chemistry 2016 |

