Characteristics and mechanism of styrene cationic polymerization in 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid
Lu Hanab,
Yi-bo Wu*ab,
Yang Danab,
Hao Wangab,
Xiao-qian Zhangab,
Xu-ling Weic,
Wen-li Guoab and
Shu-xin Liab
aDepartment of Material Science and Engineering, Beijing Institute of Petrochemical Technology, Beijing, 102617, China. E-mail: wuyibo@bipt.edu.cn
bBeijing Key Lab of Special Elastomeric Composite Materials, Beijing, 102617, China
cPetrochemical Research Institute, Petrochina, 100195, China
First published on 31st October 2016
Abstract
There has recently been a wide range of trends in the use of ionic liquids (ILs) as reaction solvent for various polymerization processes. A series of cationic polymerizations of styrene in 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim][PF6]) had been achieved using a variety of coinitiators combined with a,a-dimethylbenzyl chloride (CumCl) as a initiator at −15 °C. Experiments were carried out to observe the influence of solvent polarity and viscosity on polymerization rate. Compared with those in organic solvents, the cationic polymerization of styrene in [Bmim][PF6] proceeded in a milder exothermic manner. The end-group structures of the polystyrenes were identified by 1H NMR spectroscopy and MALDI-TOF-MS. The corresponding elementary reactions and mechanism of styrene cationic polymerization in [Bmim][PF6] were also discussed.
Introduction
Living/controlled cationic polymerization, which was discovered by Higashimura and Kennedy in the mid 1980s, represents an attractive technique for the synthesis of well-defined polymers with different microstructure and macroscopic properties. These polymer materials, such as butyl rubber and poly (styrene-b-isobutylene-b-styrene) thermoplastic elastomers, play an important role in the automobile industry, medical equipment, aerospace and other fields, and have irreplaceable advantages. However, halogenated alkane (e.g. methyl chloride) which usually used as solvents in cationic polymerization cause greatly environmental pollution due to its toxicity, volatile and corrosivity, and there by it is imperative to replace these solvents. In addition, various Lewis acids (e.g., BF3, SnCl4, TiCl4, AlCl3OBu2, and AlCl3)1–5 were used as coinitiator in cationic polymerization. It is difficult to separate these Lewis acid catalysts from the reaction products, and reuse/disposal of these catalysts is also a big challenge to industry.6Developing much cleaner reaction routes will play an important role to replace the tradition cationic polymerization. Ionic liquids are a class of chemicals that have recently emerged as alternatives to environmentally damaging volatile organic compounds. Now more researchers applied these new-style “green” alternatives to achieve polymer products owing to their outstanding properties, such as negligible vapor pressure, low melting points, good thermal stability, tunability, chemical inertia. Apart from these significant advantages, ILs can also dissolve several inorganic and organometallic compounds allowing several catalytic processes to be conducted under homogeneous conditions.7–9 IL is regarded as the ideal medium of cationic polymerization in chemical industry, which can be recycled and no pollution to the environment. The applications of ionic liquids have great theoretical and practical significance for the development of environment-friendly and low-energy consumption. The use of ILs as solvents for polymerization reactions has been reported for radical polymerization,10–13 polycondensation,14–16 electrochemical polymerization,17 and biochemical synthesis.18 But to date there has been little study on the application of ILs in ionic polymerization. Now the developed air- and water-stable ILs, such as 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim][PF6]),19–22 trihexyltetradecylphosphonium bis(trifluoromethanesulfonyl)amide ([P6,6,6,14][NTf2]),23 1-octyl-3-methylimidazolium tetrafluoroborate ([Omim][BF4]),24 and N-butyl-N-methyl pyrrolidinium bis(trifluoromethanesulfonyl)amide ([C4mpyr][NTf2]),23 have been successfully applied in cationic polymerization. Exploring the mechanism of cationic polymerization in ILs is a primary issue. In our team, cationic polymerizations of isobutyl vinyl ether (IBVE) in an ionic liquid 1-octyl-3-methylimidazolium tetrafluoroborate ([Omim][BF4]) had been examined at 0 °C and the polymerization mechanism of cationic polymerization in [Omim][BF4] system had been proposed by Pro Wu, et al.24 Based on these experiences, Dr Zhang finished the cationic polymerization of p-methylstyrene (p-MeSt) in imidazolium-based [NTf2−] ionic liquids and completed the further research.25
ILs are composed of positively and negatively charged ions. It is well known that cationic processes are strongly affected by the nature of the solvent. As a result of their ionic nature, ILs are regarded as polar but non-coordinating solvents with a high charge density;26,27 thus, they do not behave as simple solvents for ionic polymerization. In order to understand the ion environment and its effect on cationic polymerization, we comprehensively compared the cationic polymerization of styrene in ILs with those in organic solvents employing a series of initiating systems. The IL chosen for this experiment is 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim][PF6]), which obtained by the feature measurement of the various ILs. The main objective of the present study was to propose the characteristics and elementary reactions of styrene cationic polymerization in [Bmim][PF6] ionic liquid.
Experimental
Materials
Ionic liquid, 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim][PF6], purity > 99.0%), 1-hexyl-3-methylimidazolium hexafluorophosphate ([Hmim][PF6], purity > 99.0%), 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim][BF4], purity > 99.0%), 1-octyl-3-methylimidazolium tetrafluoroborate ([Omim][BF4], purity > 99.0%), were purchased from Shanghai Chemical Co. They were dried and degassed under vacuum at 60 °C for a few days to remove moisture. Karl-Fischer analysis of the ionic liquid indicated that the water content was reduced to less than 30 ppm. Styrene (St) (Beijing Chemical Co., 99.9%) and a,a-dimethylbenzyl chloride (CumCl, J&K SCIENTIFIC LTD. purity > 97.0%) were distilled twice over calcium hydride under reduced pressure. Dichloromethane (DCM, Beijing Chemical Co., 99.9%) was freshly distilled twice over calcium hydride under N2 atmosphere before use. Commercially available titanium tetrachloride (TiCl4, Aldrich, 99.9%), boron trifluoride etherate complex (BF3OEt2, Aldrich, purity > 98%), ethylaluminium sesquichloride (Al2Cl3Et3, Aldrich, 0.4 M solution in hexane) and anhydrous methanol (Beijing chemical Co., purity > 99.9%) were used as received. The 2-chloro-2,4,4-trimethylpentane (abbreviated as TMPCl) was synthesized according to the literature.28Polymerizations
The polymerization of styrene in ionic liquids was carried out under a dry nitrogen atmosphere ([H2O] < 0.5 ppm; [O2] < 10 ppm) in MBraun 150-M glovebox, which was equipped with a cold bath device. 50 mL screw-cap vials were used as polymerization reactors with an IKAMS3 vortex stirrer. A series of typical cationic polymerizations were carried out in [Bmim][PF6] at −15 °C using the following different initiating system. Into a 50 mL screw-cap vial 5 mL of [Bmim][PF6], 2 mL of styrene were added and mixed thoroughly at −15 °C. The polymerization was started by addition of TiCl4, BF3OEt2 and Al2Cl3Et3 respectively. The obtained polymers are insoluble in IL medium. So the polymerization is heterogeneous. After a predetermined time, the polymerization was quenched with excess prechilled methanol. The quenched reaction mixtures were washed with methanol to remove the ionic liquid. The polymer products were dried in a vacuum oven at 30 °C to a constant weight overnight. The monomer conversion was determined gravimetrically.Measurements
The number-average molecular weight (Mn) and molecular weight distribution (MWD; i.e., Mw/Mn) of the polymers were determined with a Waters 515-2410 gel permeation chromatography (GPC) system equipped with four Waters styragel columns connected in the following series: 500, 103, 104, and 105 at 30 °C. Tetrahydrofuran (THF) was used as eluent at a flow rate of 1.0 mL min−1 at room temperature. The columns were calibrated against standard polystyrene samples. The measurements were carried out at room temperature.NMR spectroscopy of the polymers was performed on a Bruker AV600 MHz spectrometer using CDCl3 as a solvent at 25 °C. 1H-NMR spectra of solutions in CDCl3 were calibrated to tetramethylsilane as internal standard (δH = 0.00).
MALDI-TOF-MS (matrix assisted laser desorption ionization time-of-flight mass spectrometry) analysis was performed on a Ultraflex (AB Sciex, America) time-of-flight mass spectrometer, equipped with a 337 nm, 50 Hz N2 laser, a delayed extraction, and a reflector. The apparatus was operated at an accelerating potential of 20 kV in reflected mode. The polymer solution (1 μL of 10 g L−1 in THF) was mixed with 10 μL of matrix solution (DHB (2,5-dihydroxybenzoic acid), 20 g L−1 in THF). The spectra were recorded in reflector mode in the absence of any cationizing agent. The final solution (1 μL) was deposited onto the target and dried in air at room temperature before irradiation. The mass spectra represent averages over 250 consecutive laser shots. External calibrations were performed with peptide calibration standard (Bruker Daltonics, Brenem, Germany). Al and Ti contents were determined by ICP Atomic Emission Spectrometry (ICP-AES) using an OPTIMA-2000DV equipment.29
Results and discussion
Selected ionic liquids
The solubility issue of the reagents in ionic liquids often plagues the efficiency of polymerization reactions. To start with, the solubility of styrene in different ionic liquids was examined first prior to cationic polymerization. In order to meet the requirements of low temperature for cationic polymerization, we mainly focused on the low-melting-point ionic liquids. In Table 1, solubility data of styrene in different ionic liquids were presented, in which [Bmim][PF6] possessed the highest solubility for styrene at difference temperatures. Low temperature can stabilize the activity center, reduce the chain transfer and chain termination reaction in cationic polymerization. However, the lower the temperature gives, the higher the viscosity of ionic liquid. Although [Bmim][PF6] has compatibility with styrene at −20 °C, the mixture became a semi-flowable and sticky liquid. As a result, we selected [Bmim][PF6] at −15 °C to investigate the feature of styrene cationic polymerization.| Ionic liquid | Solubility in ionic liquid at different temperature | ||||
|---|---|---|---|---|---|
| 15 °C | 0 °C | −5 °C | −10 °C | −15 °C | |
| [Bmim][PF6] | 0.67 | 0.63 | 0.61 | 0.60 | 0.58 |
| [Hmim][PF6] | 0.62 | 0.46 | 0.39 | 0.37 | <0.1 |
| [Bmim][BF4] | 0.35 | 0.30 | 0.22 | <0.1 | <0.1 |
| [Omim][BF4] | 0.36 | 0.29 | <0.1 | <0.1 | <0.1 |
The characteristics of styrene cationic polymerization in IL
In cationic initiating system, Lewis acid activates initiator to provide a cation and a complex counterion. The counterions chaperon the growing carbocations to form ion-pairs during the initiation and propagation reaction. The ion environment may affect the balance of ion pairs in the cationic polymerization owing to the high polarity and high charge density of IL. In this work, we studied the different initiating systems for styrene cationic polymerization in [Bmim][PF6]. Usually, the initiating systems for styrene and its derivatives include H2O, TMPCl and CumCl as initiator, and BF3OEt2, TiCl4 and Al2(Et)3Cl3 as coinitiator, The Lewis acidity of coinitiators is in the following order: Al2(Et)3Cl3 > TiCl4 > BF3OEt2. As shown in Table 2, the monomer conversion decreased with increasing the Lewis acidity no matter what initiator was used. Apparently, more chain transfer and chain termination reactions took place easily owing to the formation of loose ion pairs for the strong Lewis acid such as Al2(Et)3Cl3 in IL. Considering that the effect of polymerization by the initiator, styrene cationic polymerization initiated by water exhibited lower yield and lower Mn compared with CumCl and TMPCl. It was speculated that the low initiating efficiency might occur by the formation of hydrogen bonding for water in the imidazole-based ionic liquid. CumCl had a better solubility in IL compared with TMPCl. Therefore, CumCl was selected as the initiator to investigate the feature styrene cationic polymerization in IL.| Entry | Initiator | Coinitiator | Yield (%) | Mn × 10−4 (g mol−1) | Mw/Mn |
|---|---|---|---|---|---|
| 1 | H2O | BF3OEt2 | 63.24 | 0.588 | 1.84 |
| 2 | H2O | TiCl4 | 72.72 | 0.824 | 1.95 |
| 3 | H2O | Al2(Et)3Cl3 | 20.65 | 0.904 | 2.15 |
| 4 | CumCl | BF3OEt2 | 98.45 | 0.864 | 1.97 |
| 5 | CumCl | TiCl4 | 89.20 | 1.153 | 2.02 |
| 6 | CumCl | Al2(Et)3Cl3 | 41.74 | 1.301 | 2.20 |
| 7 | TMPCl | BF3OEt2 | 89.67 | 0.764 | 1.87 |
| 8 | TMPCl | TiCl4 | 87.50 | 1.096 | 1.89 |
| 9 | TMPCl | Al2(Et)3Cl3 | 23.75 | 1.220 | 2.10 |
In order to further understand the ion environment and its effect on cationic polymerization, we compared the characteristics of styrene cationic polymerization in organic molecule medium. Kinetic plots of styrene polymerization were performed in IL and dichloromethane under the various initiating systems, as shown in Fig. 1. A high monomer conversion (>60%) was observed in 5 minutes due to the fast rate of styrene polymerization in dichloromethane system. The monomer conversion gradually increased with prolonging polymerization time no matter what coinitiator was used. The conversion can reach to 80% after 20 minutes. Comparing with that in IL, there is the same phenomenon in CumCl/TiCl4 and CumCl/BF3OEt2 initiating systems. However, for the CumCl/Al2(Et)3Cl3 systems, a low monomer conversion and the lack of long-lived active species were observed.
Fig. 1C shows the semilogarithmic plot of ln([M0]/[M]) versus time of cationic polymerization reactions carried out using CumCl/TiCl4 and CumCl/BF3OEt2 initiating systems. Theoretically, this semilogarithmic plot should be linear for a truly living polymerization system. However, in our case, these plots deviated from linearity with using CumCl/TiCl4 and CumCl/BF3OEt2 initiating systems. Generally, the curvature in semilogarithmic plot indicates that the concentration of propagating and reversibly terminated chains decreases more rapidly than the formation of new chains through the slow initiation as in the case of cationic polymerization of styrene with TMPCl/TiCl4/dimethyl acetamide system.30 We used the initial slopes of these plots to calculate the initial apparent rate constants. Clearly, the initial polymerization rate in dichloromethane was similar to that in IL. The [Bmim][PF6] had a considerably higher normalized solvent polarity ENT as compared to dichloromethane.26 Generally, the higher the polarity of solvent gives, the faster the reaction rate. However, the high viscosity of ionic liquid may reduce the diffusion rate of monomer and thus slow down the reaction rate. Therefore, the similar polymerization rate should be attributed to combined effects of viscosity and polarity.
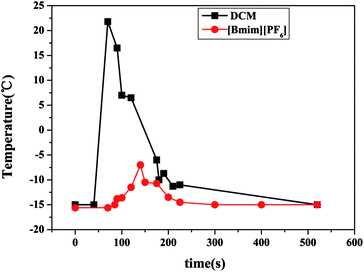
The temperature changes during the cationic polymerization in IL and dichloromethane were monitored, as shown in Fig. 2. The polymerization in dichloromethane system proceeded in a highly exothermic manner. The exothermic peak reached to 21.8 °C from −15 °C operating temperature in CumCl/TiCl4 initiating system. However, the exotherm in [Bmim][PF6] was only −7 °C with ∼90% monomer conversion. The specific heat capacity of ionic liquid [Bmim][PF6] is 1.080 kJ (kg K)−1, which is higher than DCM (0.992 kJ (kg K)−1). The milder reactions in ionic liquid may be due to the relatively higher heat capacity of the ionic liquid. The relatively higher heat capacity of ionic liquid could absorb more heat during the cationic polymerization.
Terminal structures of the polystyrenes
In the process of styrene cationic polymerization in [Bmim][PF6], the Bmim+ cation or PF6− anion may take part in initiation or termination reactions. For the sake of realizing elementary reaction of cationic polymerizations in [Bmim][PF6], the terminal structures of polystyrene were examined by 1H-NMR spectroscopy. Fig. 3 shows a typical 1H-NMR spectrum of polystyrene obtained in IL. In addition to the large absorption of methylene (g) and methine (h) of polystyrene units, a small peak at 0.99 ppm corresponding to a (CH3)2(C6H5)C– head group (f) is detected in the spectrum. Obviously, CumCl initiation takes place in [Bmim][PF6]. In general, living cationic polymerizations of styrene/vinyl monomers are terminated by adding prechilled methanol. So, polystyrene polymers obtained via a kind of chain transfer/termination usually have four possible end groups. These are: (i) a methoxide (–OCH3) end group formed by the covalent attachment of –OCH3 to the propagating carbocationic end, (ii) halogen atom as the end group, (iii) an olefinic end group and (iv) an indanyl ring as the end group.31 | ||
| Fig. 3 1H NMR spectrum of polystyrene obtained with CumCl/Lewis acid in [Bmim]PF6 at −15 °C after quenching the polymerization with methanol. (A) Al2Et3Cl3 (B) BF3 (C) TiCl4. | ||
As shown in Fig. 3, the expansion of olefinic region shows two signals at 5.3 and 5.8 ppm for exo-olefin end groups which formed by the chain termination reaction through β-hydrogen elimination reaction.32 The characteristic resonance at δ = 4.25 ppm was assigned to the halogenic chain end, that is to the propagating carbocation in connection with the counterion. The characteristic resonance at 4.0 ppm originates from the methine group resulting from terminal indanyl ring formation by the Friedel–Crafts reaction between the propagating carbocation and the phenyl group of the penultimate repeating unit.33 Also, the polymer chains terminated by adding ethanol gave a characteristic resonance of methoxide (–OCH3 δ = 3.0–3.85 ppm), which formed by the covalent attachment of –OCH3 to the propagating carbocationic end.5 Apparently, all the four end groups were found in polystyrene no matter what initiating system was used in IL.
In order to further identify the chain ends, we have analyzed MALDI-TOF spectra of final product. As Fig. 4 shows, the single series of signals are separated by 104 m/z units which equal to the molar mass of the styrene. A major peak series correspond to polymer chains bearing a methoxide (Mn = 2524 g mol−1) and halogen (Mn = 2546 g mol−1) end groups. A minor peak series, located at Mn = 2510 g mol−1, was ascribed to the chains bearing a double bond or indanyl end groups (these groups cannot be distinguished because m/z values are identical).34 These results are in good agreement with 1H NMR data which indicated that four kinds of chain transfers/chain terminations occurred in IL.
 | ||
| Fig. 4 Expanded part of the MALDI-TOF spectrum of polystyrene synthesized by the CumCl/BF3 initiating system in [Bmim][PF6] at −15 °C. | ||
The terminal structures of the polystyrenes obtained in dichloromethane with different initiating systems were also examined by 1H NMR spectroscopy, as shown in Fig. 5. Relative content of terminal structures were shown in Table 3 with comparing to that obtained in IL. Results presented in Table 3 indicated that the main terminal structure of polystyrene obtained in dichloromethane was olefinic end group. However, regardless of which Lewis acid was used as coinitiator in IL, the end of the formation of polymer structure is mainly indanyl ring and halogen, which occupied a very large proportion.
 | ||
| Fig. 5 1H NMR spectrum of polystyrene obtained with CumCl/Lewis acid in DCM at −15 °C after quenching the polymerization with methanol. (A) Al2Et3Cl3 (B) BF3 (C) TiCl4. | ||
| Run | Lewis acid | Solvent | Double bond (%) | Halogen (%) | Indanyl ring (%) | Methoxide (%) |
|---|---|---|---|---|---|---|
| 1 | Al2Et3Cl3 | [Bmim][PF6] | 10.84 | 36.76 | 50.19 | 2.21 |
| 2 | BF3 | [Bmim][PF6] | 2.52 | 40.91 | 55.65 | 0.92 |
| 3 | TiCl4 | [Bmim][PF6] | 10.35 | 34.06 | 51.76 | 3.83 |
| 4 | Al2Et3Cl3 | DCM | 39.62 | 26.12 | 26.65 | 7.61 |
| 5 | BF3 | DCM | 81.8 | 8.3 | 7.8 | 2.1 |
| 6 | TiCl4 | DCM | 43.68 | 20.38 | 17.72 | 18.22 |
Tacticity
Stereoregularity of the product polymers was revealed from the peaks of methylene carbons of the main chains in the 13C-NMR spectra recorded in CDCl3.35–39 The isotactic content of the polymers produced in [Bmim][PF6] were in the range of 38–42% (in Fig. 6), which were lower than those obtained in dichloromethane system (45–48%). It could be concluded that the propagating carbocation in [Bmim][PF6] system was much less sterically hindered to restrict the direction of the insertion of monomer molecules into the propagating carbocation. Therefore, the ionic environment may affect the interaction between propagating carbocation and metal halide-based counterion.40The polymerization mechanism of styrene in IL
On the basis of above observation, we proposed the corresponding elementary reactions of styrene cationic polymerization in [Bmim][PF6] system, as shown in Scheme 1. Initiation reactions, Lewis acid metal halide abstracted a Cl atom from CumCl, producing a carbocation and a metal halide-based counterion. Comparing with Bmim+ cation, the electrophilic carbocation was easier to attack the styrene to initiate the polymerization. Since PF6⊖ anion was very weakly nucleophilic species, halide-based counterion was likely to interact with carbocationic growing species. Therefore, it was proposed that a part of chain termination reactions directly took place toward halide-based counterion rather than PF6⊖ anion. Comparing with organic solvent, these may exist interaction between growing carbocation with PF6⊖ anion in ionic liquid which led to the interaction between the growing carbocation and counteranion become weaker. So, another chain termination reaction directly took place toward Friedel–Crafts reaction between the propagating carbocation and the phenyl group of the penultimate repeating unit, rather than β-hydrogen elimination reaction.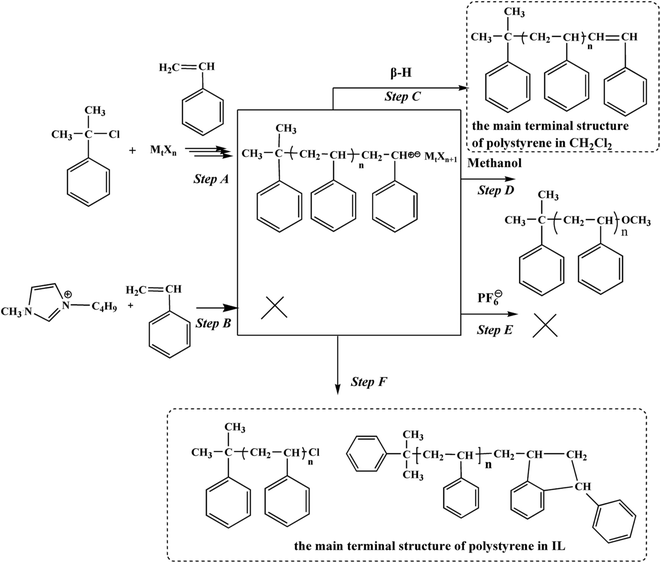 | ||
| Scheme 1 Possible polymerization pathway of cationic polymerization of styrene with CumCl/Lewis acid in [Bmim][PF6] at −15 °C after quenching the polymerization with methanol. | ||
The sterical hindrance of counteranion influenced the insertion of monomer molecules into the propagating carbocation. Thus, the interaction between propagating carbocation and metal halide-based counterion was directly responsible for stereoregulation. Due to the existence interaction between growing carbocation with PF6⊖ anion in ionic liquid, the interaction between the growing carbocation and counteranion become weaker. So, sterical hindrance of counteranion was reduced in [Bmim][PF6] which led to lower stereoregulation.
Thermal characterization
The typical DSC traces of polystyrene prepared by cationic polymerization were provided in Fig. 7a. It was showed that Tg of the polymers synthesized in IL and DCM were similar, 102.60 and 101.69 °C respectively. The thermal stabilities of polystyrene prepared in DCM and [Bmim][PF6] were shown in Fig. 7b. The polymers in IL were fairly stable up to over 356.46 °C, and the complete decomposition occurred at around 430 °C. The results revealed high thermal stability for these polymers synthesized in IL. | ||
| Fig. 7 (a) DSC traces of polystyrene synthesized in DCM (A) and in [Bmim][PF6] (B); (b) TGA curves of polystyrene synthesized in DCM (A) and in [Bmim][PF6] (B). | ||
Residual metal contents
The residual metal in polymers could affect its physical and mechanical properties, such as aging properties. The Al and Ti elemental contents in samples were determined by ICP-AES technique. As shown in Table 4, the Al and Ti elemental contents in polystyrenes obtained in IL were significantly less than that obtained in organic solvents. Polystyrenes obtained in organic solvents were firstly dissolved in dichloromethane and then washed three times by water. The results of Al and Ti elemental contents were relatively close to that obtained from IL without washing. So it was easier to separate these Lewis acid catalysts from the reaction products in IL. It was attributed to IL could dissolve several inorganic and organometallic compounds.Conclusion
The polystyrenes were successfully synthesized in an ionic liquid [Bmim][PF6] using a variety of coinitiators at −15 °C. The yield, molecular weight, and polymerization rate of the cationic polymerization were examined and compared with those in an organic solvent. It was found that initial polymerization rate in IL was similar to that in dichloromethane which resulting from interactions between viscosity and polarity factors of ILs. Styrene cationic polymerization proceeded in a milder exothermic manner in [Bmim][PF6] IL than in a traditional organic solvent. The terminal structure of polystyrene analyzed by 1H-NMR spectroscopy and MALDI-TOF spectra which clearly indicated that main chain termination reactions in IL directly took place toward halide-based counterion or toward Friedel–Crafts reaction, rather than β-hydrogen elimination reaction.Acknowledgements
This work was supported by the National Science Foundation of China (No. 51573020, 51373026 and 51503019.), Beijing Natural Science Foundation (No. 2162014), Innovation Promotion Project of Beijing Municipal Commission of Education (TJSHG201310017034), Youth Backbone Personal Project of Beijing (No. 2014000020124G085) and Project of Petrochina (No. PRIKY14041).References
- L. B. Zhang, Y. X. Wu, P. Zhou, Y. Guan, W. T. Yang and S. Ding, Chin. J. Polym. Sci., 2011, 29, 360–367 CrossRef CAS
.
- Y. Li, Y. X. Wu and L. H. Liang, Chin. J. Polym. Sci., 2010, 28, 55–62 CrossRef CAS
.
- Y. H. Peng, Chin. J. Polym. Sci., 1999, 17, 459–464 Search PubMed
.
- T. Higashimura, M. Kamigaito, M. Kato, T. Hasebe and M. Sawamoto, Macromolecules, 1993, 26, 2670–2673 CrossRef CAS
.
- S. V. Kostjuk, A. Y. Dubovik, I. V. Vasilenkol, V. P. Mardykin, L. V. Gaponik, F. N. Kaputsky and L. M. Antipin, Polym. Bull., 2004, 52, 227–234 CrossRef CAS
.
- J. H. Clark and D. J. Macquarrie, ChemInform, 1998, 29, 853–860 Search PubMed
.
- A. Figoli, T. Marino, S. Simone, E. D. Nicolo, X. M. Li, S. Tornaghi and E. Drioli, Green Chem., 2014, 16, 4034–4059 RSC
.
- G. Cevasco and C. Chiappe, Green Chem., 2014, 5, 2375–2385 RSC
.
- M. H. Kowsari and M. Fakhraee, J. Chem. Eng. Data, 2015, 60, 551–560 CrossRef CAS
.
- K. Hong, H. Zhang, J. W. Mays, A. E. Visser, C. S. Brazel, J. D. Holbrey, W. M. Reichert and R. D. Rogers, Chem. Commun., 2002, 13, 1368–1369 RSC
.
- S. Brusseau, O. Boyron, C. Schikaneder, C. C. Santini and B. Charleux, Macromolecules, 2011, 44, 215–220 CrossRef CAS
.
- H. Zhang, Y. Zhang, W. Liu and H. J. Wang, Appl. Polym. Sci., 2008, 110, 244–252 CrossRef CAS
.
- A. J. Carmichael, D. M. Haddleton, S. A. F. Bon and K. R. Seddon, Chem. Commun., 2000, 14, 1237–1238 RSC
.
- E. I. Lozinskaya, A. S. Shaplov and Y. S. Vygodskii, Eur. Polym. J., 2004, 40, 2065–2075 CrossRef CAS
.
- S. Zhang, L. D. Goncalves, H. Lefebvre, M. Tessier, B. Rousseau and A. Fradet, ACS Macro Lett., 2012, 1, 1079–1082 CrossRef CAS
.
- Y. S. Vygodskii, E. I. Lozinskaya and A. S. Shaplov, Macromol. Rapid Commun., 2002, 23, 676–680 CrossRef CAS
.
- Y. Li, Q. Qiang, X. Zheng and Z. Wang, Electrochem. Commun., 2015, 58, 41–45 CrossRef CAS
.
- S. V. Muginova, A. Z. Galimova, A. E. Polyakov and T. N. Shekhovtsova, J. Anal. Chem., 2010, 65, 331–351 CrossRef CAS
.
- M. Baśko, T. Biedroń and P. Kubisa, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 5251 CrossRef
.
- T. Biedro, P. Przemys and A. Kubisa, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 3230–3235 CrossRef
.
- M. Baśko, T. Biedroń and P. Kubisa, Macromol. Symp., 2006, 240, 107–113 CrossRef
.
- C. Bueno, V. F. Cabral, L. Cardozo-Filho, M. L. Dias and O. A. C. Antunes, J. Supercrit. Fluids, 2009, 48, 183–187 CrossRef CAS
.
- R. Vijayaraghavan and D. R. Macfarlane, Macromolecules, 2007, 40, 6515–6520 CrossRef CAS
.
- Y. B. Wu, L. Han, X. Q. Zhang, J. Mao, L. F. Gong, W. L. Guo, K. Gu and S. X. Li, Polym. Chem., 2015, 6, 2560–2568 RSC
.
- X. Q. Zhang, W. L. Guo, Y. B. Wu, L. F. Gong, W. Li, X. N. Li, S. X. Li, Y. W. Shang, D. Yang and H. Wang, Polym. Chem., 2016, 7, 5099 RSC
.
- C. Reichardt, Green Chem., 2005, 7, 339–351 RSC
.
- M. J. Earle, B. S. Engel and K. R. Seddon, Aust. J. Chem., 2004, 57, 149–150 CrossRef CAS
.
- S. Hadjikyriacou, A. Metin Acar and R. Faust, Macromolecules, 2004, 37, 351–359 CrossRef
.
- C. Li, Y. Liu, P. Cao, J. He, Z. Shi, L. Ning, J. Ao and B. Chu, Polym. Bull., 2014, 71, 2543–2557 CrossRef CAS
.
- A. N. Frolov, S. V. Kostjuk, I. V. Vasilenko and F. N. Kaputsky, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 3736–3743 CrossRef CAS
.
- S. Banerjee, T. K. Paira, A. Kotal and T. K. Mandal, Polymer, 2010, 51, 1258–1269 CrossRef CAS
.
- M. Zsuga, R. Faust and J. P. Kennedy, Polym. Bull., 1989, 21, 273–280 CrossRef CAS
.
- K. Verebélyi and B. Iván, Polymer, 2012, 53, 3426–3431 CrossRef
.
- R. Faust and J. P. Kennedy, Polym. Bull., 1988, 19, 35–41 CrossRef CAS
.
- A. Kanazawa, S. Kanaoka and S. Aoshima, Macromolecules, 2009, 42, 3965–3972 CrossRef CAS
.
- I. M. Zaleska, M. Kitagawa, S. Sugihara and I. Ikeda, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 5169–5179 CrossRef CAS
.
- S. Sugihara, M. Kitagawa, Y. Inagawa, I. M. Zaleska and I. Ikeda, Polym. Bull., 2010, 64, 209–220 CrossRef CAS
.
- M. Ouchi, A. Masami Kamigaito and M. Sawamoto, Macromolecules, 1999, 32, 6407–6411 CrossRef CAS
.
- A. Kanazawa, A. Shokyoku Kanaoka and S. Aoshima, J. Am. Chem. Soc., 2007, 129, 2420–2421 CrossRef CAS PubMed
.
- F. Feil and S. Harder, Macromolecules, 2003, 36, 3446–3448 CrossRef
.
| This journal is © The Royal Society of Chemistry 2016 |