A gold nanoparticle chemically modified gold electrode for the determination of surfactants†
Daniele Merli*,
Chiara Ferrari,
Elisa Cabrini,
Giacomo Dacarro,
Piersandro Pallavicini* and
Antonella Profumo
University of Pavia, Department of Chemistry, Via Taramelli 12, 27100 Pavia, Italy. E-mail: daniele.merli@unipv.it; piersandro.pallavicini@unipv.it
First published on 2nd November 2016
Abstract
A chemically modified electrode (CME) bearing gold nanoparticles assembled onto a gold electrode via a 1,4-benzenedimethanethiol SAM bridge has been successfully employed for the determination of surfactants in solution, using a redox probe and exploiting the tendency of surfactants to be adhere to the high-energy nanoparticles' surface.
Introduction
Chemically modified electrodes (CMEs) can be pre-designed to introduce specific interactions between the modified electrode surface and the analytes to be determined for sensitive analytical applications.1Recently self-assembled monolayer (SAM) based electrodes as tools for voltammetric determination of organic and inorganic compounds have been of increasing interest. These electrodes have several advantages over the ordinary ones, such as good reproducibility, easy preparation, exclusion of toxic compounds, such as mercury, possibility of introducing selective functional groups to bind specific compounds and great stability.2
Because of these advantages, in recent years the use of CMEs based on the chemical adsorption of thiols on gold electrodes has greatly increased, due to their easy preparation and use. Among them and relevantly to this paper, CMEs on a gold surface have been described that were first modified with a dithiol and then with gold nanoparticles via interaction with the dithiol SAM, acting as a bridge.3–6 One of the most interesting observed characteristics of gold nanoparticles is their ability to enhance the redox properties of the electrode/solution interface.7 Moreover, gold nanoparticles have high conductivity, large surface area and catalytic properties,8 that suggest their use for electroanalytical determinations of a great number of analytes. These premises lead to the preparation of a gold CME with nanoparticles that behaves as electrochemical immunosensor3 and biosensor, for example for the determination of glucose basing on a glucose oxidase reaction.4 These papers have also evidenced the ability of gold nanoparticles of ameliorating the electronic transfer rate between the electrode and an electrochemical probe in solution. Gu et al.6 have prepared a similar device capable of immobilizing haemoglobin over a layer of gold nanoparticles, anchored on a cysteamine-modified electrode, and to observe on it the electrocatalyzed reduction of hydrogen peroxide.
Surfactants can be electrochemically determined with a variety of techniques,9 e.g. by absorption onto flat gold electrodes.10 A recent example for the determination of cetylpyridinium bromide employs a boron-doped electrode modified with electrochemically-deposited gold nanoparticles, overcoated with a thiol monolayer.11 At this regard, it has to be mentioned that surfactants strongly interact with gold nanoparticles, due to the high surface energy of the latter. This property has been exploited for various applications, among which the synthesis of elongated gold nanoparticles, whose growth is driven by the specific or preferential adhesion of surfactants to certain faces of Au nanocrystals.12–14 This observation suggested us to exploit such strong tendency of surfactants to adhere to gold nanoparticles surfaces in order to detect the former in a solution, i.e. for the electroanalytical determination of surfactants. At this purpose, in this paper we present a CME consisting of a gold electrode surface that was functionalized with nude spherical gold nanoparticles (Np) via a 1,4-benzenedimethanethiol SAM bridge. The electrochemical behaviour of cationic, anionic, zwitterionic and non-ionic surfactants was investigated at the CME surface in the presence of the redox probe [Ru(NH3)6]2+/3+. None of the examined surfactants gives any response (neither faradic or capacitive) on the bare gold electrode in the potential range considered, and also they do not affect the qualitative electrochemical behaviour of the redox probes. However, specific variations in the probe peak current intensities were observed, that allow the analytical determination of the surfactants.
Experimental
Materials and reagents
Reagents of the purest grade available were purchased from Sigma-Aldrich and used as received. All the solutions: 1,4-benzenedimethanethiol, AuCl3, trisodium citrate; surfactants were prepared by Milli-Q water. The following surfactants were investigated: sodium dodecylsulfate (SDS) and sodium sulphosuccinate (anionic surfactants); benzalkonium chloride (BC) and cetyltrimethylammonium bromide (CTAB; cationic surfactants); nonylphenol ethoxylates (NE = Tergitol®), Tween 20® and Tween 100® (non-ionic surfactants); lauryl sulfobetaine (LSB, zwitterionic compound). Solutions of [Ru(NH3)6]2+/3+ (5 mM [Ru(NH3)6]Cl3 in KNO3 0.1 M as supporting electrolyte) were weekly prepared, while surfactant diluted solutions were daily prepared.Methods and instrumentation
Electrochemical measurements were carried out with AMEL Model 4330 Voltammetric Analyzer Potentiostat/Galvanostat controlled by VA peak software for AMEL, equipped with a gold electrode (BASi, geometrical area 0.020 cm2), modified as below described, as working electrode; an Ag/AgCl/NaCl (3 M NaCl, saturated with AgCl) reference electrode and a platinum wire as auxiliary electrode, both obtained from BASi.Throughout the paper, the standard deviation is reported between parentheses and refers to the uncertainty of the last digit. Mean values, unless otherwise specified, are calculated for n = 3. All measurements and constants reported in the manuscript refer to 25 °C.
Dynamic light scattering (DLS) and zeta potential measurements were obtained on the undiluted Np suspension with a Malvern Zetasizer NanoSeries nano-ZS90 instrument controlled by Zetasizer software.
Gold nanoparticles preparation and characterization
All glassware was immersed in aqua regia for 30 minutes before use (HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 3
HNO3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), then washed with Milli-Q water, and finally three times immersed in Milli-Q water and sonicated for 10 minutes. The gold nanoparticles were synthesized according to Turkevich method:15 5 mL of a 1.7 × 10−2 M solution of trisodium citrate was added to 100 mL of a 2.5 × 10−4 M solution of HAuCl4 at its boiling point under stirring. The heating was then stopped and the two solutions were left under vigorous stirring for two and a half hours. The gold nanoparticle suspension obtained was analyzed by dynamic light scattering (DLS) and transmission electron microscopy (TEM).
1), then washed with Milli-Q water, and finally three times immersed in Milli-Q water and sonicated for 10 minutes. The gold nanoparticles were synthesized according to Turkevich method:15 5 mL of a 1.7 × 10−2 M solution of trisodium citrate was added to 100 mL of a 2.5 × 10−4 M solution of HAuCl4 at its boiling point under stirring. The heating was then stopped and the two solutions were left under vigorous stirring for two and a half hours. The gold nanoparticle suspension obtained was analyzed by dynamic light scattering (DLS) and transmission electron microscopy (TEM).
For TEM characterization, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 diluted suspension of Np (with Milli-Q water) was prepared, and 10 μL of the solution was deposed on a Parlodion® membrane with copper grids; water was let evaporate at ambient temperature in a desiccator and the grids imaged with a JEOL JEM-1200 EX II instrument.
100 diluted suspension of Np (with Milli-Q water) was prepared, and 10 μL of the solution was deposed on a Parlodion® membrane with copper grids; water was let evaporate at ambient temperature in a desiccator and the grids imaged with a JEOL JEM-1200 EX II instrument.
Gold electrode pre-treatment
The electrode gold disk cross section exposed (diameter 2.0 mm) was abraded with successively finer grades alumina (from 1 μm to 0.05 μm), rinsed with water, and briefly cleaned in an ultrasonic bath to remove any trace of alumina from the surface. As abrasion favours oxidation of gold surface (as can be noticed by cyclic voltammetry), an electrochemical cleaning is performed before the SAM preparation, by cycling the potential between 0.0 V and +1.40 V in 0.5 M H2SO4 solution, with a scan rate of 200 mV s−1, until Au oxidation and reduction peak currents reach a constant value (20 cycles are usually enough).1,4-Benzenedimethanethiol SAM
The gold surface was modified by dipping the bare electrode overnight in a solution containing 5 mM 1,4-benzenedimethanethiol in ethanol. The modified electrode was then abundantly rinsed with ethanol and Milli-Q water to remove physically adsorbed dithiol molecules.4It is reported in literature16 that the considered thiol forms a perpendicularly oriented monolayer onto gold when a methanolic or ethanolic solution is used for the assembly and immersion time is not prolonged over several days, while multistrates are formed with non-polar solvents such as n-hexane and prolonged immersion times (65 h).16 The presence of a SAM of the first type (perpendicularly oriented) is essential for the assembling of the Np on the SAM.
Gold nanoparticle CME (NpCME)
The 1,4-benzenedimethanethiol SAM was dipped in a suspension of gold nanospheres (prepared as described before) overnight at 4 °C, as suggested by Gu et al.6 The modified electrode was then abundantly rinsed with Milli-Q water before use.Electrodes characterization
Before any modification, bare gold electrode area was estimated electrochemically by cyclic voltammetry (CV),17 using 1 mM ferrocene (in acetonitrile containing 0.1 M tetrabutylammonium perchlorate); an Ag/AgCl/3 M NaCl reference electrode (BASi) was used. The anodic and cathodic peak currents of the ferrocene redox couple were plotted as a function of the square root of the scan rate (in the range 10–500 mV s−1). The electrode area was calculated according to the modified form of the Randles–Sevcik equation.17 NpCME area was determined in the same manner.The SAM and NpCME electrodes were characterized with usual electrochemical techniques, following a general, widely used approach.18 In detail, the presence of the monolayer was checked by measuring the double layer capacitance of the electrode before and after SAM formation; this parameter is related to the effective thickness, the dielectric constant, and the order degree of the SAM,17,18 as it strongly depends on the presence of functional groups able to influence the dielectric constant of the monolayer.19,20 The working conditions were: potential scan from 0 mV to +300 mV, in 0.1 M KCl, at different scan rates (typically from 10 mV s−1 to 200 mV s−1); the differences between the anodic and cathodic charging currents, measured at 150 mV, were plotted vs. the scan rates, and the double layer capacitance is obtained from the slope of the linear regression, divided by twice the electrode area;14 this approach produces results virtually unaffected by faradaic contributions.
The variation of the capacitance for different immersion times in the 1,4-benzenedimethanethiol solution was used to follow the adsorption kinetics of the thiol onto the gold substrate,19 while the measurement of interfacial capacitance provides a convenient way for evaluating the degree of surface coverage, θ, of the organic adsorbates, evaluating the capacitance, Ct, at any time t, and the capacitance of the fully covered monolayer.21,22 Surface coverage (Γ, mol cm−2) can be estimated by reductive desorption of thiol from the electrode surface. The film desorbs in alkaline (pH > 11) solution through a reductive monoelectronic reaction.23
The values of the charge necessary for desorbing the film was determined by integrating the area under the cathodic wave (Ep = −950 mV) in the ip–E curves obtained in 0.5 M KOH (Ei = +200 mV, Ef = −1400 mV, scan rate 100 mV s−1), after compensating for charging current.
The response of the modified electrode to hexaamminoruthenium(III) that behaves as reversible electroactive marker on gold surfaces,24,25 was evaluated by CV, at 100 mV s−1 scan rate in a solution 5.0 mM [Ru(NH3)6]Cl3 (0.1 M KNO3, pH 7.0, Ei = −200 mV, Ef = +400 mV).
Electron transfer rate (k°) has been evaluated for the redox marker Fe(CN)64−/3− by CV (K4Fe(CN)6 5 mM in 0.1 M KNO3, pH 7.0, Ei = 0 mV, Ef = +700 mV, scan rate 100 mV s−1), applying the following equation:26,27
k° = 2.18(DαnFv/RT)1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[−α2nF/RT(ΔE)] exp[−α2nF/RT(ΔE)]
| (1) |
AFM (atomic force microscopy) characterization of Np modified gold surface
The AFM was conducted on a surface simulating the NpCME. The gold surface was obtained by sputtering gold onto a microscopy cover glass slide (22 mm × 22 mm, thickness 0.13–0.16 mm) with a Scancoat Six Sputter Coater, Edwards; the following operative conditions were used: deposition time 8 minutes; applied voltage 1.5 kV; current 25 mA; pressure 5.5 × 10−1 mbar. The thickness of the layer is 300 nm. The gold nanoparticle CME was assembled on this surface as previously described. AFM imaging was carried out on a Thermo Microscopes CPII AFM, operated in tapping mode with a NT-MDT silicon tip NSG01. Data analysis was carried out with the Image Processing and Data Analysis software – version 2.1.15 by TM Microscopes.Response of the SAM electrode towards surfactant – DPV
The considered surfactants are not electroactive in the potential ranges at which the NpCME is stable (from −600 to +600 mV vs. Ag/AgCl/3 M NaCl) but they affect the electrochemical behaviours of the two redox probes at this electrode. So the modification of the redox marker current and/or potential in the presence of different amounts of the surfactants was investigated.The response at the NpCME of [Ru(NH3)6]2+/3+ (5 mM in 0.1 M KNO3 as supporting electrolyte) was investigated in the presence of different surfactants at various concentrations, by differential pulse voltammetry (DPV, scan rate 50 mV s−1), in the potential range between −500 mV and +300 mV. The surfactant adsorbs on the nanoparticles, decreasing the response toward the electrochemical probe depending on the electrostatic interactions and on the formation of a coating on the electrode that hinders the electron transfer toward the redox probe. The variation of the redox probe current was found to be proportional to the amount of surfactant in solution. The signal obtained in absence of the surfactant (ib) with respect to the decrease of the redox marker current at any surfactant addition (ic), divided for the current ib, is plotted vs. concentration of the surfactant; the value is reported in the text as Δi/ib = (ib − ic)/ib.
The calibration curve is prepared by registering a DPV of the [Ru(NH3)6]2+/3+ probe at a fixed concentration (5 mM), followed by additions of increasing concentration of the surfactant to the cell (standard addition method). In the same way, for the analysis of an unknown sample, first a DPV of [Ru(NH3)6]2+/3+ in the supporting electrolyte alone was recorded, and then in presence of the sample also containing 5 mM [Ru(NH3)6]3+, to maintain constant its concentration. The variation of the current is quantified by the standard addition method, by plotting (Δi/ib = (ib − ic)/ib) vs. surfactant standard additions. All measurements were performed with a scan speed of 50 mV s−1.
To evaluate mutual interferences of the different surfactants, i.e. for determining the influence of nonylphenol ethoxylate (NE) over SDS, similar DPV experiments were performed. In detail, after the registration of voltammogram of the solution containing ruthenium, 0.0175 mM SDS was added and a new DPV was recorded. In the following voltammograms, additions of NE and SDS at the same concentration (0.0175 mM) were alternated until the concentration of each surfactant was 0.175 mM. In more detail, the first surfactant was added to the solution (e.g. 1 mg L−1), and the voltammogram registered before the addition of the same amount (e.g. 1 mg L−1) of the second surfactant, followed by the registration of a new voltammogram. The sequence was repeated with single additions (e.g. 1 mg L−1) of each surfactant, separately. The analogous procedure was used for the evaluation of the interference of each surfactant over the others considered (SDS, LSB, BC).
Preparation of synthetic samples
Three synthetic samples each containing a different surfactant (anionic, cationic, neutral), depicting galenic formulations inscribed in Farmacopea Ufficiale Italiana and reported in standard galenic formulation textbooks were prepared and analysed. A galenic formulation contained sulfamerazine 2% as active ingredient, the anionic surfactant sodium sulphosuccinate 0.15%, and other inert ingredients.28Two other samples both containing BC as preservative were analyzed; one is a nasal decongestant containing phenylephrine hydrochloride 2.5%, BC 0.100% and inert ingredients, and the other one is a commercially available nasal spray containing beclomethasone dipropionate 0.5 mg mL−1 as active ingredient, BC 0.100%, and inert ingredients.
Finally the last preparation contained dextromethorphan hydrobromide as active ingredient (antitussive) at 2.5%, NE 0.3% and inert ingredients.28
Results and discussions
Gold nanoparticles
Spherical gold nanoparticles were prepared according to described methods.15 The mean diameter of the nanoparticles evaluated on three different TEM images is 17.2(±3.6) nm, 18.1(±3.6) nm and 17.4(±3.7) nm (see ESI1†). We take 17.6 nm as the average diameter. Accordingly, the surface area of each particle is 9.73 × 10−12 cm2. TEM analysis was also confirmed by DLS data, showing dimensional monodispersion and sharp dimensional distribution with a comparable average value (d = 16 nm). The zeta potential of the nanoparticles was −33(1) mV when analysed in the same solution in which they were prepared (pH = 4.7), without dilution of the sample, and it is predictive of long term stability. The negative value is expected as the nanoparticles are surrounded by citrate ions.29 Also from a qualitative point of view, the suspension appears stable and no turbidity was observed even after 1 month of storage at room temperature or at 4 °C.SAM and NpCME characterization
Bare gold electrode area was calculated by cyclic voltammetry (CV), as described in the experimental. Briefly, we used 1 mM ferrocene in acetonitrile (0.1 M tetrabutylammonium perchlorate) with an Ag/AgCl/3 M NaCl as reference electrode. The anodic and cathodic peak currents of the ferrocene redox couple were plotted as a function of the square root of the scan rate and the electrode area was obtained according to the modified form of the Randles–Sevcik equation.14 Fig. 1A, blue profile, shows a representative CV at a 100 mV s−1 scan rate. The bare electrode area was estimated to be 0.0202(5) cm2 (n = 5, α = 0.05), in accordance with the value geometrically calculated (0.020 cm2). When the bare electrode was coated with 1,4-benzenedimethanethiol, the active area dropped to ∼0 cm2 (no significant CV signal). When the AuNP overlayer was formed on the dithiol monolayer, the NpCME electrode recovered an active area of 0.0152 cm2 (Fig. 1A, red, for a CV at a 100 mV s−1 scan rate). The smaller area indicates that not all the surface of the electrode is covered with nanoparticles. The mean number of nanoparticles on the electrode surface can be estimated by dividing the effective area of the CME (0.0152 cm2) by the surface area of each nanoparticle (i.e. the calculated 9.73 × 10−12 cm2 value), obtaining ∼1.56 × 109. Dividing the number of nanoparticles by the surface area of the whole electrode (0.0202 cm2), a surface density of 7.73 × 1010 nanoparticles per cm2 is obtained. Further characterization carried out by Atomic Force Microscopy (AFM) confirms such picture (ESI2†).The capacitance of the electric double layer for the NpCME was 5.4(2) μF cm−2, the one of the 1,4-benzenedimethanethiol SAM 4.0(3) μF cm−2 and that of bare gold electrode, in the same experimental conditions, 61(2) μF cm−2. The presence of the gold nanoparticles contributed to the capacitance of the NpCME, intermediate between that of the nude bare gold surface and the one of SAM, behaving as a dielectric, relatively free of defects, uniformly packed and oriented.30 1,4-Benzenedimethanethiol, as other members of the same class of compounds (thiols), adsorbs onto the gold surface at a rate proportional to the free surficial sites, following a Langmuir kinetic (see ESI3†), to form a highly ordered and oriented monolayer.31,32
The high surface coverage (Γ) obtained, 1.94 × 10−9 mol cm−2, corresponding to 1.21 × 1015 thiol molecules for cm2, is in good agreement with literature data33 and confirms the expected highly ordered SAM structure.17 Considering the calculated number of gold nanoparticles per cm2 (9.41 × 1010 cm−2), for each nanoparticle there are at least ∼104 available thiol molecules. On the other hand, it is obviously not expected that 104 thiol groups bind a single nanoparticle. The obtained monolayer is not very densely packed, as a sphere with d = 17.6 nm projects an area of 2.43 × 10−12 cm2 on a flat surface. Thus, 7.73 × 1010 particles in one cm2 occupy a maximum area of 0.188 cm2, i.e. 18.8% of the available space. Moreover, noble metal nanoparticles, when lying on a molecular adhesive SAM, are only partially immersed in the molecular layer (34% in the case of AgNP of comparable dimensions on a mercaptopropylsilane monolayer).34 When using the reversible redox marker [Ru(NH3)6]2+/3+, we observed that the CV curve of the SAM changed drastically with respect to bare gold, passing from an electrochemically reversible (ΔEp = 60 mV) to a capacitive, quasi-reversible shape (ΔEp approx 400 mV), see Fig. 1B. This is due to the sluggish electron-transfer kinetics through the dithiol film.35 Coherently, at the SAM the currents were strongly reduced (ca. 10% of the current on bare gold). However, when gold nanoparticles were assembled on the dithiol film, a CV with a ΔEp of 60 mV was obtained, so that a reversible behaviour was restored4 (Fig. 1B). These results are better than those obtained with gold nanoparticles grafted with alkanedithiols to a gold surface.4 As a matter of fact and differently from aryldithiole,36 such molecules are flexible and can form “chelates” with the electrode without binding the nanoparticles and leading to a mixed SAM–Np surface with large islands of the electrode free from Np.37 The lower capacitive currents, and the better defined waves obtained at NpCME, lead to a favourable signal-to-noise ratio, with the faradic/capacitive current ratio ∼2.5 for bare gold and ∼5 for NpCME, with the redox probe [Ru(NH3)6]2+/3+. Peak potentials were shifted toward more negative potentials (E1/2 shifted from −60 mV to −170 mV) and the oxidation of [Ru(NH3)6]2+/3+ was favoured, in agreement with results already observed in literature for gold nanoparticles.38,39 These results evidenced the ability of the nanoparticles, grafted to the electrode through the dithiol bridge, to facilitate the redox reaction at the interface,4 while the SAM acted as a (partial) shield towards electron transfer.21 These results are also supported by the electron transfer rate k° toward Fe(CN)63−/4−, that resulted to be 1.7 × 10−3 cm s−1 for the NpCME, significantly higher than k° at bare gold electrode (3.18 × 10−4 cm s−1) and at SAM (4.2 × 10−6 cm s−1), that are within the values reported in literature.40,41
Response of the SAM electrode to surfactant – DPV
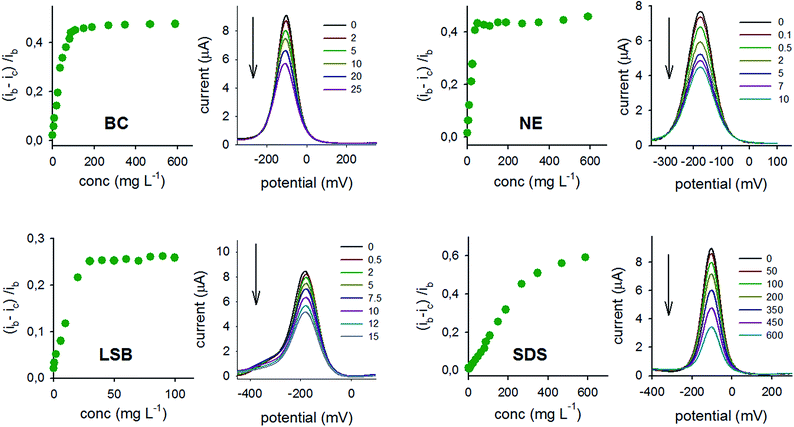
The methodology is based on the variation of the current of a redox marker (at a fixed concentration) in absence and presence of the surfactant. It must be stressed that the behaviour observed is characteristic of the Np system, as no proportional variation of the current vs. surfactant concentration is observed in the same experimental conditions at the bare gold electrode and at the SAM (see ESI†). The increased tendency of small spherical gold nanoparticles to adsorb surfactants on their surface is involved in this case.12–14 Surfactants adsorb of course also on flat gold surfaces and studies have been carried out on such electrodes, showing that surfactant adsorption depends on the potential applied or on the nature of the electrolyte salt, also evidencing the role played by applied potential and electrolyte concentration in manipulating the size and shape of the surfactant aggregates on the electrode surface.42 Determining or even hypothesizing the shape of the aggregates of the adsorbed surfactants on the Au NP surface was outside the scope of this paper. However we minimized the variations of the relevant parameters by using in all experiments a constant concentration of the same electrolyte (0.1 M KNO3) and in a limited potential range (−400 mV to +300 mV vs. Ag/AgCl/3 M NaCl). The results observed occurred at the Np surface, as no micelles are expected in solution, being the critical micellar concentrations (CMC) of the surfactants employed higher than the concentrations used here, at least up to the current plateaux. CMC for SDS is around 2000 mg L−1;43 CMC for BC is 20 mg L−1,44 and for LSB is 670 mg L−1.45 CMC for NE is 60 mg L−1, according to Dow Chemicals specifications for their Tergitol™ NP-9 surfactant: in this latter case we used NE concentrations both higher and lower than its CMC, but finding that the current value of the redox marker vs. NE concentration (see Fig. 2) already reached a plateau before the CMC value of NE. The behaviour observed is similar for various surfactants belonging to the same class. In fact, the same results obtained with BC can be obtained also with other cationic ammonium-based surfactant (e.g. CTAB), while SDS behaved as dioctyl sodium sulfosuccinate or sodium benzenesulfonate, and NE gave similar results as the other neutral surfactants tested, namely Tween 20 and Triton X-100 (see ESI5†). The results obtained in presence of 5 mM redox probe are described below and displayed in Fig. 2.BC and [Ru(NH3)6]2+/3+
The current decreases on increasing the concentration of the surfactant, up to 110 mg L−1 BC, due both to the shielding effect of the surfactant adsorbed on the surface and to the electrostatic repulsion of the positively charged species.NE or LSB and [Ru(NH3)6]2+/3+
The current decreases on increasing the concentration of the surfactants from 2 mg L−1 of NE and LSB up to 50 mg L−1 and 30 mg L−1, respectively. As no overall electrostatic interaction is expected with NE and LSB (total charge = 0), the observed current decrease is to be attributed to a shielding effect exerted by the surfactants.SDS and [Ru(NH3)6]2+/3+
The current decreases on increasing the concentration of the surfactant. This is due to a shielding effect of the surfactant adsorbed on the surface, that prevails over the attraction between the negatively charged SDS and positively charged probe. The current decreases linearly with a concentration of SDS up to 350 mg L−1, then reaching a plateau.Notably, the same response is obtained with similar molar quantities of surfactants belonging to the same class, and with a redox probe concentration in the range 1–20 mM; 5 mM concentration was chosen, as better defined peaks were observed.
It is interesting to notice that limiting (ib − ic)/ib values are sharply reached for [Ru(NH3)6]2+/3+ when BC and NE reach ∼50–60 mg L−1 concentration. A smoother, almost continuous trend for (ib − ic)/ib is instead observed in the case of LSB and in particular of SDS. The four surfactants have exact or average molecular weights in the same range (from 288.38 for SDS to ∼338 for BC) so their mass concentrations can be directly compared instead of molar concentrations. They are linear molecules in the case of SDS and LSB while they contain an aromatic ring in the case of NE and BC. The latter may favor a compact, ordinate SAM formation on the Au NP with a similar surface density, thanks to π–π interactions and to the large polar (NC) and charged (BC) heads, resulting in the sharp ascending-plateau trends observed. Moreover, in the case of SDS the continuous decrease of ic (and thus the (ib − ic)/ib increase) with no flat plateau even at 350 mg L−1, may be attributed to a further shielding effect exerted by weak electrostatic absorption on the oppositely charged probe complex.
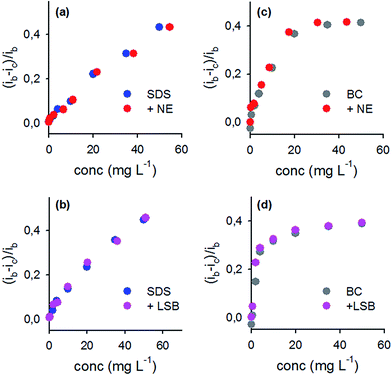
Mutual interference of each surfactant over the variation of the probes' signal
These tests were performed with the aim of evaluating the influence, over the probe's current, of surfactants belonging to different classes and simultaneously present in the sample. Anionic and zwitterionic surfactants cause a decrease of the signal of the probe, although to different extent.According to the results obtained and shown in Fig. 3 it can be concluded that the decrease in the current height of each probe can be due to the charged surfactant (BC or SDS), while zwitterionic and non-ionic surfactants have a negligible effect when added in equimolar quantities to the charged surfactants, in a concentration range from 0.015 mM up to 0.175 mM. The interference in the determination of charged surfactants in presence of zwitterionic/non-charged surfactants, in similar concentration, is negligible. This result is fully supported by the already reported observations that non-ionic surfactants based on a polyethyleneoxide hydrophilic components46 (like NE) and zwitterionic surfactants (like LSB)47 interact weakly with the surface of gold nanoparticles, compared to charged ones.
Surfactant determination by DPV with [Ru(NH3)6]2+/3+
For every surfactant class, calibration curves were performed by standard addition method, in presence of [Ru(NH3)6]2+/3+. Calibration curves obtained are: SDS, Δi/ib = 0.02(4) mg L−1 + 0.001(2), R2 = 0.9716 (8 standards in the range 0.1–15 mg L−1); NE, Δi/ib = 0.031(2) mg L−1 − 0.01(2), R2 = 0.9789 (8 standards in the range 0.1–10 mg L−1); BC, Δi/ib = 0.0442(1) mg L−1 + 0.001(2), R2 = 0.9972, (9 standards from 0.5 mg L−1 to 20 mg L−1); LSB, Δi/ib = 0.00323(2) mg L−1 + 0.0002(1), R2 = 0.9915 (8 standards from 0.1 mg L−1 to 15 mg L−1). In all cases, LOD of 0.03 mg L−1 and LOQ of 0.1 mg L−1 were found. Results obtained applying the described analytical method to real or simulated samples, show a standard deviation of 10–15% and recovery in the range 93–120%, as reported in Table 1.| Sample | Present surfactant | Found |
|---|---|---|
| a Material and methods section for the preparation and characteristic.b Standard deviation. | ||
| Sulfamerazine 2%, oral solutiona | Dioctyl sodium sulfosuccinate 0.15% | 0.18% (s.d.b 15%) recovery: 120% |
| Dextromethorphan hydrobromide linctusa 2.5% | NE 0.3% | 0.28% (s.d.b 11%) recovery: 93% |
| Phenylephrine HCl 2.5% nasal dropsa | BC 0.100% | 0.103% (s.d.b 10%) recovery: 103% |
| Beclomethasone dipropionate 0.5 mg mL−1, nasal spraya | BC | 0.095% (s.d.b 10%) |
Interferences
The interferences due to common excipients present in pharmaceutical formulation were evaluated; in particular, it was found that sugar, mannitol, inorganic salts (such as NaCl, KCl, Na2SO4, NaNO3) did not interferes also at a concentration ratio larger than 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with respect to the surfactant and EDTA interferes only at concentration >10
1 with respect to the surfactant and EDTA interferes only at concentration >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, causing a decrease of the ruthenium probe current. The active principles illustrated in Table 1 (sulfamerazine, dextromethorphan, phenylephrine), when added as pure compounds did not interfere even at >100
1, causing a decrease of the ruthenium probe current. The active principles illustrated in Table 1 (sulfamerazine, dextromethorphan, phenylephrine), when added as pure compounds did not interfere even at >100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 concentration with respect to the surfactant. Common preservatives used in addition to BC (such as Nipagin) did not interfere at concentration >10
1 concentration with respect to the surfactant. Common preservatives used in addition to BC (such as Nipagin) did not interfere at concentration >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with respect to the surfactant.
1 with respect to the surfactant.
Conclusions
The preparation of a CME based on gold nanoparticles assembled onto a gold electrode via a 1,4-benzenedimethanethiol SAM bridge is described. Surfactants adsorb onto gold NpCME, modifying the current response of the redox active probe [Ru(NH3)6]2+/3+ depending on their overall charge. The variation in the probe peak current is quantitatively dependent on the surfactant concentration. The interaction described is peculiar of the gold nanoparticles grafted to the electrode surface, as no variation of the probe current was observed on bare gold, also at surfactant concentrations larger than those that produce a discernible signal onto NpCME (e.g. at tens of mg L−1 with respect to the 0.1 mg L−1 necessary at NpCME). Through representative examples of real pharmaceutical preparations, we have also demonstrated that sensitive surfactants determination in pharmaceutical samples can be performed, and the method is useful for anionic, cationic, zwitterionic or non-ionic surfactants. The analysis is not time consuming as no pre-treatment of the sample is required, and has fair sensitivity, despite a larger standard deviation with respect to commonly used HPLC methods.48,49 With respect to other proposed electrochemical (voltammetric or potentiometric) determination methods,9 the method we propose does not use mercury, and LOD and LOQ are significantly better than those already reported, that are in the range 1–5 mg L−1. Other devices presents comparable LOD11 but their use seems to be limited to cathionic surfactants, while the proposed method can be used both for anionic, cationic and non-ionic surfactants. Moreover, with respect to these already reported electrochemical methods we have deeply investigated the application to real samples, while the literature methods only rely on pure solution of surfactants. Possible interferences in real samples were accurately investigated. LOQ of 0.1 mg L−1 was observed for all surfactants. The simultaneous presence of compatible surfactants (neutral or zwitterionic over cationic and anionic) was investigated, and it was shown that the charged (cationic or anionic) species can be determined also in presence of neutral or zwitterionic species without significant interference also at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 molar ratio. Common excipients (sodium chloride, sugars) and some active compounds usually present in pharmaceutical formulations did not interfere in the determination, excluding EDTA at a concentration ratio greater than 10 with respect to the surfactant. Finally, the method requires the use of relatively cheap materials.
10 molar ratio. Common excipients (sodium chloride, sugars) and some active compounds usually present in pharmaceutical formulations did not interfere in the determination, excluding EDTA at a concentration ratio greater than 10 with respect to the surfactant. Finally, the method requires the use of relatively cheap materials.
Acknowledgements
University of Pavia is kindly acknowledged for the financial support to Elisa Cabrini (Fondo Ricerca Giovani 2015).Notes and references
- J.-M. Zen, A. Senthil Kumar and D.-M. Tsai, Electroanalysis, 2003, 15, 1073–1087 CrossRef CAS.
- R. C. Alkire, D. M. Kolb, J. Lipkowski and P. N. Ross, Chemically Modified Electrodes, Wiley-VCH, Weinheim, 2009, vol. 11 Search PubMed.
- J. Zhou, L. Du, L. Zou, Y. C. Zou, N. Hu and P. Wang, Sens. Actuators, B, 2014, 197, 220–227 CrossRef CAS.
- S. Zhang, N. Wang, H. Yu, Y. Niu and C. Sun, Bioelectrochemistry, 2005, 67, 15–22 CrossRef CAS PubMed.
- J. Zhang, Y. Liu, J. Lv, Y. Cao and G. Li, Microchim. Acta, 2015, 182, 281–288 CrossRef CAS.
- H. Y. Gu, A. M. Yu and H. Y. Chen, J. Electroanal. Chem., 2001, 516, 119–126 CrossRef CAS.
- D. Huang, F. Liao, S. Molesa, D. Redinger and V. Subramanian, J. Electrochem. Soc., 2003, 150, G412–G417 CrossRef CAS.
- http://www.sigmaaldrich.com/materials-science/nanomaterials/gold-nanoparticles.html.
- M. Gerlache, J. M. Kauffmann, G. Quarin, J. C. Vire, G. A. Bryant and J. M. Talbot, Talanta, 1996, 43, 507–519 CrossRef CAS PubMed.
- D. Bizzotto, V. Zamlynny, I. Burgess, C. A. Jeffey, H.-Q. Li, J. Rubinstein, Z. Galus, A. Nelson, B. Pettinger, A. R. Merill and J. Lipkowski, Amphiphilic and ionic surfactants at electrode surfaces, in Interfacial electrochemistry, theory, experiment and applications, ed. A. Wieckowski, Marcel Dekker, New York, 1999 Search PubMed.
- Y. Wang, J. Zhi, Y. Liu and J. Zhang, Electrochem. Commun., 2011, 13(1), 82–85 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Adv. Mater., 2001, 13(18), 1389–1393 CrossRef CAS.
- P. Pallavicini, G. Chirico, M. Collini, G. Dacarro, A. Donà, L. D'Alfonso, A. Falqui, Y. Diaz-Fernandez, S. Freddi, B. Garofalo, A. Genovese, L. Sironi and A. Taglietti, Chem. Commun., 2011, 47, 1315–1317 RSC.
- P. Pallavicini, A. Donà, A. Casu, G. Chirico, M. Collini, G. Dacarro, A. Falqui, C. Milanese, L. Sironi and A. Taglietti, Chem. Commun., 2013, 49, 6265–6267 RSC.
- J. Turkevich, P. C. Stevenson and J. Chiller, Discuss. Faraday Soc., 1951, 11, 55–75 RSC.
- S. W. Joo, S. W. Hang and K. J. Kim, J. Colloid Interface Sci., 2001, 240(2), 391–399 CrossRef CAS PubMed.
- D. Merli, F. Ravasio, S. Protti, M. Pesavento and A. Profumo, Talanta, 2014, 130, 90–95 CrossRef CAS PubMed.
- U. Kumar Sur, R. Subramanian and V. Lakshminarayanan, J. Colloid Interface Sci., 2003, 266, 175–182 CrossRef.
- J. A. M. Sondag-Huethorst and L. G. J. Fokkink, Langmuir, 1996, 11, 2237–2241 CrossRef.
- M. A. Pope and I. A. Aksay, J. Phys. Chem. C, 2015, 119, 20369–20378 CAS.
- I. Tredici, D. Merli, F. Zavarise and A. Profumo, J. Electroanal. Chem., 2010, 645, 22–27 CrossRef CAS.
- B. B. Damaskin, O. A. Petrii and V. V. Batrakov, Adsorption of Organic Compounds on Electrodes, Plenum, New York, 1971 Search PubMed.
- D. Merli, S. Protti, M. Labò, M. Pesavento and A. Profumo, Talanta, 2016, 151, 119–125 CrossRef CAS PubMed.
- M. M. Walczak, D. D. Popenoe, R. S. Deinhammer, B. D. Lamp, C. Chung and M. D. Porter, Langmuir, 1991, 7, 2687–2693 CrossRef CAS.
- F. Zavarise, D. Merli and A. Profumo, Anal. Chim. Acta, 2010, 668, 177 CrossRef CAS PubMed.
- A. Profumo, F. Zavarise, D. Merli, I. Tredici, G. Alberti, S. Protti and M. Fagnoni, Anal. Chim. Acta, 2007, 598, 58–64 CrossRef CAS PubMed.
- J. Klinger and J. K. Kochi, J. Phys. Chem., 1981, 85, 1731–1741 CrossRef.
- F. Bettiol, Manuale delle preparazioni galeniche, Tecniche Nuove, Milano, 2003 Search PubMed.
- C. Bustamante, J. Vesenka, C. L. Tang, W. Rees, M. Guthold and R. Keller, Biochemistry, 1992, 31, 22–26 CrossRef CAS PubMed.
- A. Tlili, A. Abdelghani, K. Aguir, M. Gillet and N. Jaffrezic-Renault, Mater. Sci. Eng., C, 2007, 27(4), 620–624 CrossRef CAS.
- D. S. Karpovich and G. J. Blanchard, Langmuir, 1994, 10, 3315–3322 CrossRef CAS.
- R. C. Thomas, L. Sun and R. M. Crooks, Langmuir, 1991, 7, 620–622 CrossRef CAS.
- C. D. Bain, E. B. Troughton, Y. Tao, J. Evall, G. M. Whitesides and R. G. Nuzzo, J. Am. Chem. Soc., 1989, 111, 321–335 CrossRef CAS.
- P. Pallavicini, C. Bernhard, G. Dacarro, F. Denat, Y. A. Diaz-Fernandez, C. Goze, L. Pasotti and A. Taglietti, Langmuir, 2012, 28, 3558–3568 CrossRef CAS PubMed.
- M. Lu, X. H. Li, B. Z. Yu and H. L. Li, J. Colloid Interface Sci., 2002, 248, 376–382 CrossRef CAS PubMed.
- A. Profumo, M. Fagnoni, D. Merli, E. Quartarone, S. Protti, D. Dondi and A. Albini, Anal. Chem., 2006, 78, 4194–4199 CrossRef CAS PubMed.
- C. Vericat, M. E. Vela, G. Benitez, P. Carro and R. C. Salvarezza, Chem. Soc. Rev., 2010, 39, 1805–1834 RSC.
- S. Yan, S. Zhang, Y. Lin and G. Liu, J. Phys. Chem. C, 2011, 115(14), 6986–6993 CAS.
- M.-C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed.
- B. Liu, A. J. Bard, C. Z. Li and H. B. Kraatz, J. Phys. Chem. B, 2005, 109, 5193–5198 CrossRef CAS PubMed.
- C. Miller, P. Cuendet and M. Gratzel, J. Phys. Chem., 1991, 95, 877–886 CrossRef CAS.
- M. Chen, I. Burgess and J. Lipkowski, Surf. Sci., 2009, 603, 1878–1891 CrossRef CAS.
- P. Mukerjee and K. J. Mysels, Critical Micelle Concentration of Aqueous Surfactant Systems, NSRDS-NBS 36, Government Printing Office, US, Washington, DC, 1971 Search PubMed.
- G. A. Georgiev, N. Yokoi, K. Koev, E. Kutsarova, S. Ivanova, A. Kyumurkov, A. Jordanova, R. Krastev and Z. Lalchev, Biochem. Mol. Biol., 2011, 52(7), 4645–4654 CAS.
- J. G. Weers, J. F. Rathman, F. U. Axe, C. A. Crichlow, L. D. Foland, D. R. Scheuing, R. J. Wiersema and A. G. Zielske, Langmuir, 1991, 7, 854–867 CrossRef CAS.
- P. Pallavicini, S. Basile, G. Chirico, G. Dacarro, L. D'Alfonso, A. Donà, M. Patrini, A. Falqui, L. Sironi and A. Taglietti, Chem. Commun., 2015, 51, 12928–12930 RSC.
- A. Casu, E. Cabrini, A. Donà, A. Falqui, Y. Diaz-Fernandez, C. Milanese, A. Taglietti and P. Pallavicini, Chem.–Eur. J., 2012, 18, 9381–9390 CrossRef CAS PubMed.
- F. Al-Rimawi, W. Zareer, S. Rabie and M. Quod, J. Pharm. Anal., 2012, 2(1), 67–70 CrossRef CAS.
- A. Marcomini, S. Capri and W. Giger, J. Chromatogr. A, 1987, 403, 243–252 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: TEM images and dimensional counting, AFM imaging, DPV electrochemical responses with different surfactants. See DOI: 10.1039/c6ra22223d |
| This journal is © The Royal Society of Chemistry 2016 |