Preparation of one-dimensional Fe3O4@P(MAA-DVB)–Pd(0) magnetic nanochains and application for rapid degradation of organic dyes†
Abstract
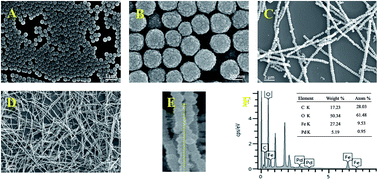
One dimensional (1D) magnetic Fe3O4@P(MAA-DVB)–Pd(0) nanochains are successfully prepared through distillation precipitation of methacrylic acid (MAA) and divinylbenzene (DVB) over Fe3O4 nanochains procured from magnetic-field-induction of hollow magnetic nanoparticles. 1D magnetic Fe3O4@P(MAA-DVB)–Pd(0) nanochains were subsequently obtained by complexation of Pd2+ and carboxyl groups on the surface of nanochains and late reduction by NaBH4. The Pd content is approximately 3.69 wt% determined by TGA, and the saturation magnetization of Fe3O4@P(MAA-DVB)–Pd(0) nanochains with average length of 20 μm is 38 emu g−1. The as-prepared nanochains can be used as a magnetic stir to catalytically reduce dyes like rhodamine B (RhB). The color of the solution fades within 30 s. Furthermore, the nanochains exhibits excellent reusability. Thus, this kind of 1D magnetic nanochains shows potential application value in ultra-fast and highly efficient degradation of dyes.

 Please wait while we load your content...
Please wait while we load your content...