Monitoring and quantification of the complex bioaccumulation process of mercury ion in algae by a novel aggregation-induced emission fluorogen†
Yusheng Jiangab,
Yuncong Chenc,
Maha Alrashdid,
Wen Luobe,
Ben Zhong Tangc,
Jihong Zhangf,
Jianguang Qin*b and
Youhong Tang *d
*d
aCollege of Aquaculture and Life Sciences, Dalian Ocean University, Liaoning 116023, China
bSchool of Biological Sciences, Flinders University, South Australia 5042, Australia. E-mail: jian.qin@flinders.edu.au; Tel: +61 8 82013045
cDepartment of Chemistry, The Hong Kong University of Science and Technology, Hong Kong, China
dCentre for Nanoscale Science and Technology, Flinders University, South Australia 5042, Australia. E-mail: youhong.tang@flinders.edu.au; Tel: +61 8 82012138
eSchool of Life Sciences, Shaoxing University, Zhejiang 312000, China
fCollege of Food Science and Engineering, Dalian Ocean University, Liaoning 116023, China
First published on 17th October 2016
Abstract
In this study, a novel methodology was developed using a specified aggregation-induced emission fluorogen (AIEgen) to monitor and quantify the complex bioaccumulation process in a microcosm aquatic ecosystem. Mercury ion (Hg2+) was used as the pollutant and Euglena gracilis as a representative algal species in water, to develop this new methodology for understanding the processes of bioaccumulation and biorelease of a heavy metal in algae. AIEgen can easily detect Hg2+ in the environment by the “turn-on” feature, and a relationship was built among photoluminescence (PL) intensity, AIEgen concentration, and Hg2+ concentration. The AIEgen was effectively used for quantifying Hg2+ concentration in the bioaccumulation process by reading the PL intensity of the solution. Bioaccumulation, bioaccumulation efficiency, and the ratio of Hg2+ in Euglena gracilis cells and the environment were carefully characterized by this novel method and the results were further validated with the existing well-established analytical method. The quantitative detection of Hg2+ absorption and release from the algae by the AIEgen demonstrates a novel, green, and sustainable approach to understand the dynamics of Hg2+ between aquatic organisms and the environment.
1. Introduction
The consequences of industrial, mining, and agricultural activities over the past century have greatly altered the natural biogeochemical cycles of potentially toxic substances.1 These toxic substances can be transferred to the upper levels of food chains through bioaccumulation in aquatic ecosystems, leading to adverse effects on human health and the environmental function.2 The magnitude of bioaccumulation in aquatic organisms depends on the length of food chain and can usually reach a multiple of 10 or more between two links on a food chain.3 Consequently, the concentration of some toxic substances in apex ocean predators such as dolphins and swordfish at the top of the food chain may be augmented by a million times compared with the surrounding environment.4Inorganic/organic species of mercury is highly toxic and is also considered one of the most dangerous and ubiquitous pollutants in water.5–7 The main source of mercury pollutants is release from burned coal, with an annual release of 4400–7500 metric tons estimated by the United Nations Environment Program (UNEP).8 The mercury ion (Hg2+) can easily pass through skin and respiratory and gastrointestinal tissues into the human body and cause damage to the central nervous system and endocrine systems.9 Moreover, Hg2+ can be converted to methyl-mercury by a variety of microorganisms in the water, further accumulating and magnifying along the aquatic food chain to disrupt the normal development of the fetal brain in humans.10
The bioaccumulation of Hg2+ in aquatic organisms is astonishing and the methods for monitoring and quantifying Hg2+ in aquatic food webs are complicated and costly. Traditional methods, such as atomic absorption/emission spectroscopy and Auger-electron spectroscopy, are limited to use in well-established labs, due to the requirement of sophisticated instrumentation and labor-intensive processes for sample preparation.11 Although, there are plenty of analytical methods, especially the electrochemical ones,6,7 can be used to determine mercury ions in well-established labs and on-site, there is still a lack of an alternative ease way by fluorescence measurement.
The recent development of fluorescent sensors in the fields of biological and environmental sciences has allowed researchers to directly visualize and monitor the complex biological processes on site. At present, various fluorescent probes based on small molecules, polymeric materials, nanoparticles, and dosimeters have served as powerful tools for biological research.12 Aggregation-induced emission fluorogens (AIEgens) have attracted considerable attention due to their highly emissive behavior of aggregated illumination.13 Most fluorogens have a problem of aggregation-caused quenching, though they are highly emissive in the solution state.14 Their weak emission or even non-emission in an aggregated state may significantly decrease their efficiency as sensitive sensors. AIEgens, on the other hand, are non-emissive in the solution state but are induced to emit intensely upon aggregate formation.15 The “turn-on” fluorescent biosensors of AIEgens permit successful application in aqueous media for biomedical imaging, diagnosis, and therapy.16 Various AIE-based chemosensing systems have been generated for a wide range of analyses for ions, explosives, fingerprints, and even gases in the fields of environmental monitoring, water quality control, homeland security, and forensic investigation.17,18
Recently, AIE-based fluorescent probes have been developed to detect Hg2+ in isolated solution,19,20 and such applications can be further explored to detect Hg2+ dynamics in the aquatic ecosystems. In this study, we used a specially designed AIEgen to understand the role of microalgae absorbing Hg2+ in water and to further quantify the Hg2+ dynamics over time in algal cells after Hg2+ absorption. A novel methodology based on this AIEgen was developed for detection and quantification of Hg2+ bioaccumulation in algae, as well as Hg2+ release from algae after bioaccumulation. This study provides a new insight in the study of toxic bioaccumulation in aquatic systems, shedding light on understanding the mechanism of Hg2+–algae interactions in aquatic systems and showing a sustainable way for Hg2+ removal from the environment by using algae as green absorbents.
2. Materials and methods
2.1. Materials
All reagents were obtained from Sigma-Aldrich (Australia) unless otherwise specified. AIEgen (m-TPE-RNS) has been synthesized and characterized previously.20 The Hg2+ detection mechanism of the AIEgen structure changes from m-TPE-RNS to m-TPE-RNO with Hg2+, as shown in Scheme 1.Stock solution of m-TPE-RNS at a concentration of 1.0 mM was prepared by dissolving the AIEgen in acetonitrile (ACN). The solution was stored in a sealed dark bottle at 4 °C before use. Stock solution of HgCl2 at a concentration of 1.0 mM was prepared by dissolving HgCl2 in Milli Q water and was stored in a sealed bottle at 4 °C before use.
Euglena gracilis were obtained from the School of Biological Sciences, Flinders University, with the mean length of 15–20 μm and diameter of 8–10 μm. The culture medium was prepared by mixing 30 g wheat grains, 25 g rice grains and 5 g skim milk powder into 1 L Milli Q water. After autoclaving at 121 °C for 5 min, the culture medium was stored at 4 °C. Euglena gracilis were inoculated into the culture medium at 10% (v/v) and cultured in 250 mL Erlenmeyer flasks at 24 °C in a temperature-controlled room under continuous light (70 μmol photons per m−2 s−1). The flask was stirred manually twice a day to prevent algal settlement. The Euglena gracilis cells were counted on a haemocytometer (Improved Marienfeld Neubauer, Germany) to determine cell density.
The test solutions for AIE characteristics contained 10 μM m-TPE-RNS in ACN or water/ACN mixtures with different water fractions (0–99%). The test solutions for Hg2+ determination contained 10 μM m-TPE-RNS and various Hg2+ concentrations in the form of HgCl2. Photoluminescence (PL) intensity was read on a fluorescent spectrometer (Varian, Australia), with the excitation wavelength at 355 nm.
2.2. Time optimization for staining Hg2+ with AIE
After mixing 10 μL of the m-TPE-RNS stock solution with 390 μL ACN and 590 μL Milli Q water, 10 μL of Hg2+ stock solution was added. The PL intensity was measured at 1, 5, 15, 30, 60, and 90 min on the fluorescent spectrometer to determine the peak absorption value over time. To develop the master map for Hg2+ determination, a series of Hg2+ concentrations (0.5, 1, 5, 10, 20, and 30 μM) were detected in 1 mL of multiple AIE concentrations (0.5, 1, 5, 10, 20, and 30 μM) with the abovementioned time. After the PL intensity was obtained, the PL master map was developed to show the relationships between PL intensities with AIE and Hg2+ concentrations. The Hg2+ concentration could be quantified by the corresponding PL intensity measured by the fluorescent spectrometer.2.3. Determination of Hg2+ concentration in a solution with algae
Euglena gracilis with the density of 1.0 × 106 cells per mL were introduced to a series of water samples containing different HgCl2 concentrations (10, 20, and 30 μM). Algal cells in normal and deformed shapes were counted after 1 h from each treated sample on an optical microscope (Leica, USA) and a recovery rate was calculated after 24 h when algal cells were transferred to clean Milli Q water to determine the stress response of algal cells to Hg2+. Algae were harvested from the culture medium by centrifugation (Hettich EBA20, Australia) at 4500 rpm for 1 min. Then the amount of Hg2+ residue in the culture medium was measured by the AIE method using the fluorescent spectrometer and the PL readings were converted to Hg2+ concentrations using the developed master map.2.4. Time-dependent Hg2+ absorption and release by algae
Euglena gracilis cells were harvested from the culture medium by centrifugation, and were adjusted to a cell density of 1.0 × 106 cells per mL with Milli Q water, to which HgCl2 was added to final concentrations of 10 and 20 μM. The rate of Hg2+ absorption by algae was measured at 1, 30, 60, and 120 min. Before each measurement of Hg2+ concentration in the solution, algal cells were removed by centrifugation. Briefly, the Hg2+ concentration in 600 μL of supernatant was determined at 400 μL AIE in the ACN solution to reach the AIE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio of 3
water ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The PL intensity of the stained solution was read on the fluorescent spectrometer.
2. The PL intensity of the stained solution was read on the fluorescent spectrometer.
2.5. Algal density-dependent Hg2+ absorption and release
To determine the Hg2+ absorption by algae, a series of Euglena gracilis densities (0.2, 0.4, 0.6, 0.8, and 1.0 × 106 cells per mL) were prepared by diluting the stock algal culture with Milli Q water. HgCl2 was added to each algal sample to reach a final Hg2+ concentration of 20 μM. After incubation for 30 min, algae cells were removed by centrifugation. The Hg2+ remained in the solution was determined by the AIE method with the AIE concentration of 20 μM. The amount of Hg2+ residuals was also measured by ICP-MS (PerkinElmer NexION 300, US) for comparison with the result detected by the AIE method.To determine the Hg2+ release from algae, HgCl2 was first added to the Euglena gracilis culture (1.5 × 106 cells per mL) to reach a final concentration of 20 μM Hg2+. After absorption for 45 min, algal cells were separated from the culture medium by centrifugation. The algae cells were then rinsed with Milli Q water to remove Hg2+ attached to the outside of cells. After rinsing once, the algal cells were re-suspended in Milli Q water at a density of 6.0 × 106 cells per mL. At different time slots (3 h, 24 h, and 27 h), the algal cells were separated from the culture medium by centrifugation, and the concentration of Hg2+ released from the algae was quantified by the AIE method.
3. Results and discussion
3.1. AIE features and response to Hg2+
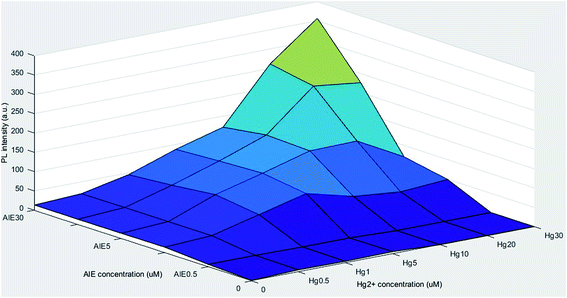
The AIE features of m-TPE-RNS were investigated in water/ACN mixtures with different water volume fractions (fw, vol%) from 0 to 99%, with the highest PL intensity occurring at the 60% fraction point.20 Therefore, the fw was fixed at 60% in water/ACN mixtures in the subsequent experiments. In the presence of Hg2+, the m-TPE-RNS displayed strong fluorescence emission under 350 nm UV light excitation, and photoexcitation at 590 nm was induced and displayed on the PL spectra.20 The fluorescent behavior of Hg2+ quantitation with the m-TPE-RNS was determined and optimized with the concentrations of AIE and Hg2+ and the interaction time between AIE and Hg2+ (see ESI Fig. S1–S3†). The PL intensities varied with those parameters and formed a complex relationship, as shown in Fig. 1. However, a linear relationship between PL intensity and Hg2+ concentration was observed when the ratio of AIE concentration and Hg2+ concentration was less than 1. As shown in Fig. S2† with AIE concentration of (a) 10 μM and (b) 20 μM, the linear relationship between PL intensity and Hg2+ concentration was established with Hg2+ concentration less than (a) 10 μM and (b) 20 μM, respectively.3.2. Algae response to Hg2+ concentrations
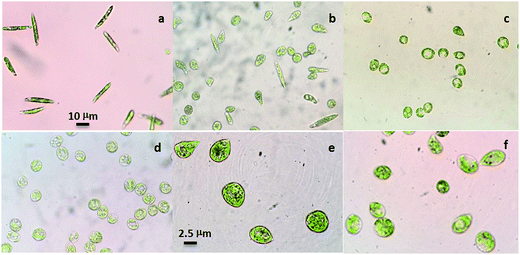
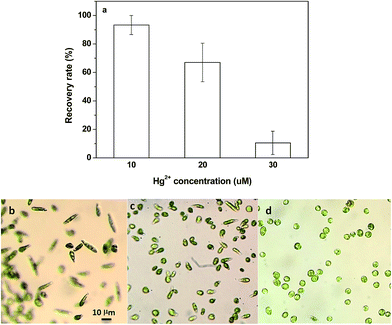
In the present work, Euglena gracilis was used to characterize Hg2+ absorption and this species has been used extensively in the laboratory as a model organism for nutrient absorption.21 This free-living flagellate belongs to a select group of organisms with proven capacity to resist and accumulate heavy metals.22 Euglena gracilis can normally swim in the culture medium in an elongated or spindle shape (Fig. 2a), and can change shape immediately in response to chemical and physical stress.23 By virtue of this special character, the effect of Hg2+ toxicity on the algal cells can be quantified. In this study, more Euglena gracilis cells contracted into a “ball” shape and became immobile with the increase in Hg2+ concentration from 0 to 30 μM. The contractile vacuole could be clearly seen in Euglena gracilis cells at Hg2+ concentrations of 10 μM and 20 μM, respectively (Fig. 2b, c, e and f). However, the contractile vacuole disappeared and the central part of the algal cells became condensed and wrinkled after treatment with Hg2+ at 30 μM (Fig. 2d).Next, an algal recovery experiment was conducted using the algal recovery ratio (mobile cells/total cells by number) to further determine the effect of Hg2+ toxicity on Euglena gracilis. Fig. 3 shows the algal recovery ratio in clean water over 24 h after incubation with different Hg2+ concentrations for 1 h. After incubation with 10 and 20 μM Hg2+, most of the algal cells were recovered to normal morphs and became motile within 24 h with the recovery ratios of 93% and 67%, respectively. However, the recovery ratio of algae cells incubated with Hg2+ at 30 μM dropped significantly to about 10%, and most of them lost chlorophyll (Fig. 3d). Therefore, Hg2+ concentrations of 10 μM and 20 μM were used in the subsequent study.
3.3. AIE quantification for bioaccumulation of Hg2+ in algae
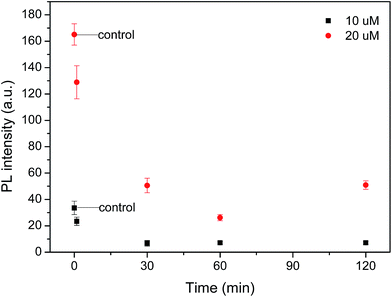
The Euglena gracilis cell lacks a cell wall. Instead, it has a pellicle made up of a protein layer supported by a substructure of microtubules.24 It has been demonstrated that the phospholipid bilayer and biological membrane are highly permeable to HgCl2 mediated by non-protein substances.25,26 In this context, the Euglena gracilis cell is similar to a sponge for Hg2+ absorption in solution, and can quickly absorb Hg2+. The reminder of the Hg2+ ions in the solution after algal absorption was quantified by the master map shown in Fig. 1. The physiology of Euglena gracilis is demonstrated in Fig. S4† and chlorophyll in the Euglena gracilis cell excited by 488 nm laser diode with a red emission was observed by a fluorescence microscope. Florescent images of Euglena gracilis cells under Hg2+ concentrations of 10 μM and 20 μM, respectively have been taken with m-TPE-RNS, as shown in Fig. S5.†Fig. 4 shows that a significant drop in PL intensity occurred at the very beginning for both Hg2+ concentrations of 10 μM and 20 μM, indicating the significant initial uptake of Hg2+ by algal cells. The PL intensity leveled off and plateaued after 1 h in both Hg2+ concentrations. Therefore, 1 h was used as the incubation time in the subsequent study.
The amount of bioaccumulation (At), bioaccumulation efficiency (Et) and bioaccumulation ratio (Rt) of Hg2+ in algae were calculated as:
| At = C0 × V0 × MHgCl2 × (1 − Pt/P0)/Bm | (1) |
| Et = At/t | (2) |
| Rt = Ct/Cm = Ct/(C0 − Ct) | (3) |
Fig. 5a shows the bioaccumulation of Hg2+ inside Euglena gracilis. The bioaccumulation was a time- and Hg2+ concentration-dependent process. In the medium with 10 μM Hg2+, the amount of Hg2+ inside algal cells increased from zero to 0.64 μg mg−1 within the first 30 min. In the medium with 20 μM Hg2+, the amount of Hg2+ in algal cells increased from zero to 1.13 μg mg−1 within the same time period. Further incubation did not enhance bioaccumulation much and the amount of Hg2+ augment inside algae did not increase significantly. For the bioaccumulation efficiency as shown in Fig. 5b, a higher Hg2+ concentration corresponds to a higher Et. However, at both 10 μM and 20 μM Hg2+ concentrations, a similar trend of Et with time was observed, in that the high Et in the first 30 min decreased to a low value between 30 and 60 min. The ratio of Hg2+ accumulation (Rt) increased to 4 within 1 h in the medium with 10 μM Hg2+ (Fig. 5c) and exceeded 5 within 1 h in the medium with 20 μM Hg2+. In other words, a total of 78% Hg2+ in the 10 μM Hg2+ medium and 84% in the 10 μM Hg2+ medium was transferred from the environment to the algal cells within 1 h. Rapid bioaccumulation of Hg2+ in algae was also reported,27 which was in the range of 1–2 μg Hg2+ per mg algae by Devars et al.28
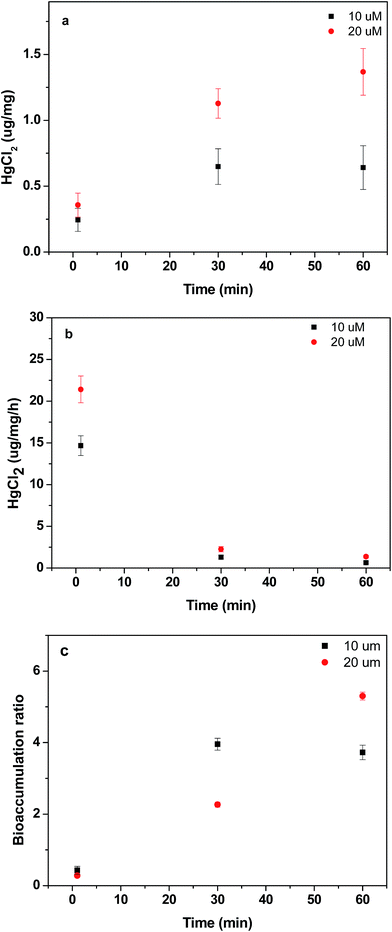 | ||
| Fig. 5 (a) Amount of bioaccumulation, (b) bioaccumulation efficiency, and (c) bioaccumulation ratio of Euglena gracilis at different Hg2+ concentrations over time. | ||
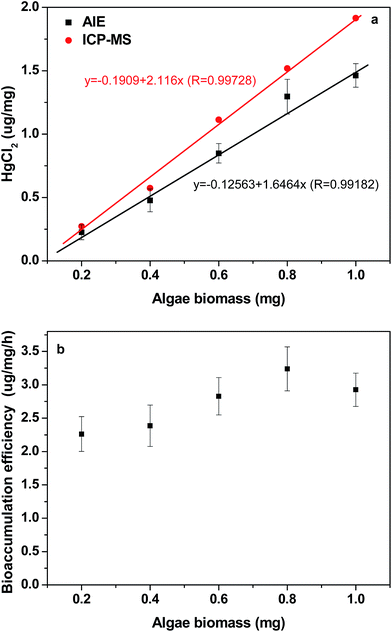
Algal density is another important parameter influencing the process of bioaccumulation (Fig. 6). With an increase in algal density, the amount of bioaccumulation increased in the algae, as shown in Fig. 6a. In the culture media with 5.62 μg mL−1 HgCl2 (20 μM), the bioaccumulation efficiency of Hg2+ increased from 2.25 μg mg−1 h−1 at the algal density of 0.2 mg algae per mL to 3.25 μg mg−1 h−1 when the algal density increased to 0.8 mg mL−1, as shown in Fig. 6b. Meanwhile, the amount of Hg2+ residuals in the culture medium was also measured through ICP-MS to verify bioaccumulation of HgCl2 by Euglena gracilis. Both methods showed an excellent matching relationship between the rate of HgCl2 bioaccumulation and algal biomass, despite a slight difference in the slope and intercept between fitting equations as shown in Fig. 6a. The ICP-MS results of Hg2+ bioaccumulation were higher than those estimated by the AIE method. The reason might be due to Hg2+ absorption by bacteria after algae were removed, as the organic carbon in the algal culture medium could facilitate bacterial proliferation.29 The relationship of competitive absorption for Hg2+ between bacteria and algae warrants further investigation.
3.4. AIE detection of biorelease of Hg2+ in algae
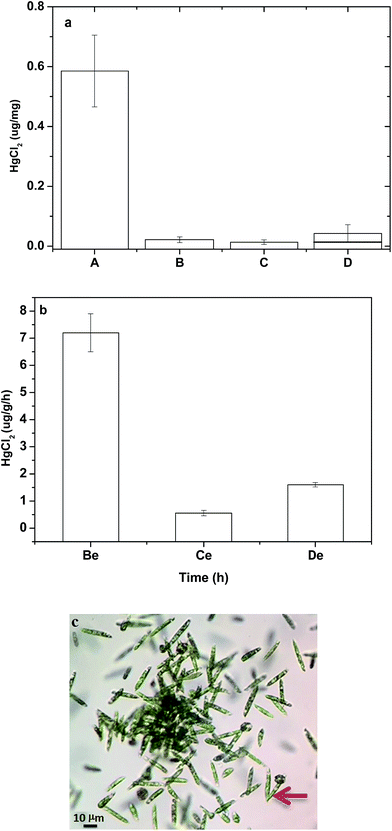
The biorelease of Hg2+ from bioaccumulated algae with Hg2+ was undertaken to demonstrate a promising way to achieve green and sustainable removal of Hg2+ by using algae. Previous results (Fig. 3a) demonstrated that 67% of algae cells were recovered from an immobile status to a mobile status when algae were incubated with 20 μM HgCl2 for 1 h and then recovered in clean water for 24 h. To further quantify Hg2+ release, algae were harvested after incubation in 20 μM HgCl2 for 45 min, and then transferred into clean water. Subsequently, the amounts of 0.02 and 0.013 μg Hg2+ per mg algae were detected in the clean water at 3 h and 24 h, respectively, after Hg2+release, as shown in Fig. 7a. Interestingly, the released HgCl2 at 24 h was less than that at 3 h. A possible reason is that the recovered algae could continue to reproduce through binary cell division, as shown in Fig. 7c. The newly produced algae would have the ability to reabsorb the Hg2+ released to the water and to further reduce Hg2+ concentration in the environment. Nevertheless, there was another interesting phenomenon observed in the mercury release assay. After mercury release for 3 h (column B in Fig. 7a), the algae were retrieved from the clean water by centrifugation and re-dispersed in a new batch of clean water. The total amount of released Hg2+ in 27 h (initial 3 h and subsequent 24 h) (column D in Fig. 7a) was greater than that of the initial release in the first 24 h (column C in Fig. 7a). A higher releasing efficiency was achieved by repeated changing of the medium during the releasing period, as shown in columns Ce and De in Fig. 7b, suggesting that a dynamic balance exists between the intracellular and extracellular environments. If a higher Hg2+ concentration gradient exists between the intracellular and extracellular environments, higher release efficiency can be achieved.In summary, after algae were recovered from Hg2+ stress within a certain period (e.g., 24 h), most of the algae were still mobile, despite Hg2+ accumulation inside algal cells. Interestingly, the algae were still capable of reproduction through binary cell division. The newly produced algae would have the ability of reabsorption from the Hg2+ released to the water to further reduce Hg2+ concentration in the environment, so algae can act as a “live” absorbent to remove Hg2+ continuously from the environment.
4. Conclusions
In this study, we demonstrated a novel and succinct method to monitor and quantify the bioaccumulation of heavy metals in the aquatic environment by a specified AIEgen. The AIEgen with Hg2+ detection and quantification abilities was designed and synthesized. The bioaccumulation process of Hg2+ in Euglena gracilis was characterized with this AIE method. This novel method can be used to track the dynamics of bioaccumulation quantitatively and also can obtain results comparable to those measured from the tedious conventional analytical method. The AIE method provides a new insight in the study of toxic bioaccumulation in aquatic systems.Acknowledgements
Y. Jiang and W. Luo are grateful for the support of the China Scholarship Council (CSC) and Shaoxing University with a visiting scholar program to Flinders University respectively. Y. Tang is grateful for the support from Flinders University through the Faculty of Science and Engineering Establishment Grant.References
- S. Lindberg, R. Bullock, R. Ebinghaus, D. Engstrom, X. Feng, W. Fitzgerald, N. Pirrone, E. Prestbo and C. Seigneur, Ambio, 2007, 36, 19–32 CrossRef CAS PubMed.
- F. A. P. C. Gobas, W. de Wolf, L. P. Burkhard, E. Verbruggen and K. Plotzke, Integr. Environ. Assess. Manage., 2009, 5, 624–637 CrossRef CAS PubMed.
- R. A. Lavoie, T. D. Jardine, M. M. Chumchal and K. A. Kidd, Environ. Sci. Technol., 2013, 47(23), 13385–13394 CrossRef CAS PubMed.
- M. F. Gutierrez, A. M. Gagneten and J. C. Paggi, Ecotoxicology, 2012, 21, 37–47 CrossRef CAS PubMed.
- E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443–3480 CrossRef CAS PubMed.
- V. Vyskočil and J. Barek, Crit. Rev. Anal. Chem., 2009, 39, 173–188 CrossRef.
- J. Gajdar, E. Horakova, J. Barek, J. Fischer and V. Vyskočil, Electroanalysis, 2016 DOI:10.1002/elan.201600239.
- J. Liu and Y. Lu, Angew. Chem., Int. Ed., 2007, 46, 7587–7590 CrossRef CAS PubMed.
- F. Di Natale, A. Lancia, A. Molino, M. Di Natale, D. Karatza and D. Musmarra, J. Hazard. Mater., 2006, 132, 220–225 CrossRef CAS PubMed.
- W. D. Atchison and M. F. Hare, FASEB J., 1994, 8, 622–629 CAS.
- Y. Gao, S. De Galan, A. De Brauwere, W. Baeyens and M. Leermakers, Talanta, 2010, 82, 1919–1923 CrossRef CAS PubMed.
- R. P. Haugland, The molecular probes handbook: A guide to fluorescent probes and labeling technologies, Life Technologies, Carlsbad, CA, 2010 Search PubMed.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441–2453 CrossRef CAS PubMed.
- R. T. K. Kwok, C. W. T. Leung, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2015, 44, 4228–4238 RSC.
- J. Mei, Y. Hong, J. W. Y. Lam, A. Qin, Y. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS PubMed.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332–4353 RSC.
- F. Guo, W. P. Gai, Y. Hong, B. Z. Tang, J. G. Qin and Y. H. Tang, Chem. Commun., 2015, 51, 17257–17260 RSC.
- G. Chen, Z. Guo, G. Zeng and L. Tang, Analyst, 2015, 140, 5400–5443 RSC.
- Y. Chen, W. Zhang, Y. Cai, Y. Hu, R. T. K. Kwok, J. W. Y. Lam, X. Gu, Z. He, Z. Zhao, X. Zheng, B. Chen, C. Gui and B. Z. Tang, J. Am. Chem. Soc., 2016 Search PubMed , under review.
- A. G. Russell, Y. Watanabe, J. M. Charette and M. W. Gray, Nucleic Acids Res., 2005, 33, 2781–2791 CrossRef CAS PubMed.
- S. Devars, R. Hernández and R. Moreno-Sánchez, Arch. Environ. Contam. Toxicol., 1998, 34, 128–135 CrossRef CAS PubMed.
- E. Stallwitz and D. P. Häder, J. Photochem. Photobiol., B, 1993, 18, 67–74 CrossRef CAS.
- E. S. Kempner, Stimulation and inhibition of the metabolism and growth of Euglena gracilis, in The biology of Euglena, ed. D. E. Buetow, Academic, New York, 1982, vol. III, pp. 197–525 Search PubMed.
- J. Gutnecht, J. Membr. Biol., 1981, 61, 61–66 CrossRef.
- L. P. Karniski, J. Biol. Chem., 1992, 26, 19218–19225 Search PubMed.
- W. Zhao, Hydrobiology, China agriculture press, 2005 Search PubMed.
- S. Devars, C. Avilés, C. Cervantes and R. Moreno-Sánchez, Arch. Microbiol., 2000, 174, 175–180 CrossRef CAS PubMed.
- S. Devars, J. S. Rodríguez Zavala and R. Moreno Sánchez, Water, Air, Soil Pollut., 2011, 216, 51–57 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra22190d |
| This journal is © The Royal Society of Chemistry 2016 |