Fabrication of high performance flexible all-solid-state asymmetric supercapacitors with a three dimensional disc-like WO3/stainless steel electrode
Abstract
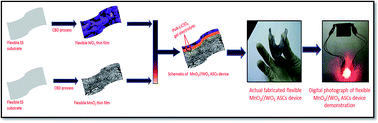
Presently, significant attention has been paid towards the rational synthesis of nanostructured anode and cathode electrode materials for assembling high-performance supercapacitors. Despite significant progress being achieved in designing cathode electrode materials, anode electrode materials with high capacitance are hardly investigated. In the present article, a tungsten oxide (WO3) thin film is prepared on a flexible stainless steel substrate by a wet chemical method and used as an anode electrode to fabricate a flexible asymmetric supercapacitor (ASC). An electrochemical investigation of the WO3 thin film shows a maximum specific capacitance of 530 F g−1 at 1 mA cm−2 in a potential window of 0 to −0.8 V in 1 M Na2SO4 electrolyte. In addition, a highly energetic, flexible ASC device is assembled using a WO3 thin film as an anode, a MnO2 thin film as a cathode and polymer gel as an electrolyte. The as-assembled MnO2//WO3 ASC device exhibited a stable electrochemical potential window of 1.8 V and better cycling stability. What's more, the flexible MnO2//WO3 ASC device achieves a high specific capacitance of 115 F g−1 with an acceptable specific energy of 52 W h kg−1 at a current density of 3 mA. Hence, the proposed flexible MnO2//WO3 ASC device creates one more option for anode materials to develop flexible energy storage devices.

 Please wait while we load your content...
Please wait while we load your content...