Acetyl salicylic acid–ZnAl layered double hydroxide functional nanohybrid for skin care application
Abstract
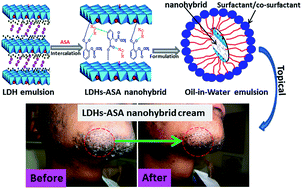
In this study, a pharmaceutically active ingredient, acetyl salicylic acid (ASA), was intercalated into ZnAl layered double hydroxide (LDH). The LDH–ASA nanohybrid material was characterized by XRD, FTIR, SEM, ICP-MS, TEM and TGA. Successful incorporation of ASA into LDH interlayers through electrostatic interactions was confirmed by FTIR results. ASA in the intercalated form showed higher thermal stability in comparison to pure ASA. In vitro release studies revealed that the nanohybrid in release media (artificial sweat/phosphate-buffered saline) could effectively facilitate the ASA diffusion by significantly increasing the release time. The nanohybrid was also used to develop a topical skin care formulation, and skin permeation kinetic studies were conducted by a Franz diffusion cell using reconstructed skin samples. The results from the Franz diffusion cell experiments showed that the formulation with the LDH–ASA nanohybrid drastically increased the penetration rate for ASA compared to the formulation with pure ASA. From these studies, we can conclude that the in situ intercalation of an LDH–ASA nanohybrid system is a promising alternative active ingredient for topical cosmetic emulsions.


 Please wait while we load your content...
Please wait while we load your content...