Biogenic and chemogenic synthesis of TiO2 NPs via hydrothermal route and their antibacterial activities
Abstract
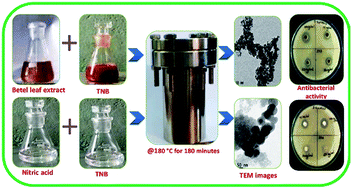
Herein, we outline a simple hydrothermal route for the synthesis of titanium dioxide (TiO2) nanoparticles (NPs) using novel biogenic source Piper betel leaf extract and chemogenic source nitric acid as capping and reducing agents. The synthesized TiO2 NPs were subjected to various characterization techniques such as UV-vis spectrophotometry, X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), transmission electron microscopy (TEM), and energy dispersive X-ray spectroscopy (EDS) for their optical, structural, morphological and compositional analysis. The obtained results reveal the following features; XRD analysis confirms the phase formation and presence of nanocrystalline TiO2. FT-IR spectroscopy analysis reveals the presence of Ti–O vibrational bands, O–H bands, plant proteins, tannins, carbohydrates and polyols. TEM analysis shows the NPs were of spherical shape with an average size of about 8 nm and 75 nm for biogenic and chemogenic synthesized TiO2 respectively. EDS analysis confirms the chemical compositions of the NPs having Ti and O elements. Further, these synthesized TiO2 NPs were studied for their antibacterial activity against multi-drug resistant microorganisms like Staphylococcus aureus (Gram-positive) and Escherichia coli (Gram-negative); here we observe that the minimum inhibitory concentration (MIC) values for biogenic and chemogenic are 25 μg mL−1 and 50 μg mL−1 respectively. These results suggest that biogenic synthesized NPs act as a more effective antibacterial agent than chemogenic synthesized NPs.

 Please wait while we load your content...
Please wait while we load your content...