Characterizing the interactions between humic matter and calcium ions during water softening by cation-exchange resins†
Abstract
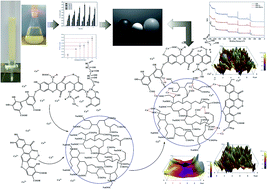
Reusing wastewater can enormously reduce environmental pollution and save water. One of the key obstacles to reuse is the scaling caused by calcium (Ca) ions and humic matter (HM). The interactions among HM, Ca ions and cation-exchange resins (CERs) during the water softening process were explored via lab-scale and pilot-scale tests. Surface topography, pore structure, and surface molecular structure of the CERs were determined using an atomic-force microscope, an automatic mercury porosimeter, a laser-Raman spectrum and X-ray photoelectron spectroscopy, respectively. The surface charge of CERs was assessed by measuring zeta potential. Experimental results showed that HM can react with Ca-ions to form HM–Ca+ and Ca ions can act as a bridge for HM and CER, suggesting that Ca ions and HM can be simultaneously removed by CERs. Kinetic analyses indicate that a pseudo-first order equation was able to accurately describe the overall adsorption kinetics and diffusion of Ca ions through a liquid film of CER was the rate-determining step for the overall adsorption process. Further investigation revealed that during the first 60 minutes of adsorption, the effect of HM on the CER adsorption kinetics can be described well by pseudo-first and pseudo-second order equations. This indicates that diffusions of Ca ions, HM, and HM–Ca+ and their interactions were equivalent for controlling the initial stage of adsorption. In addition, 99% of Ca ions and 30–60% of HM in wastewater were removed in the pilot-scale test, suggesting that CERs have significant potential to remove Ca ions and HM simultaneously in wastewater.

 Please wait while we load your content...
Please wait while we load your content...