Effects of aging on the phytochemical profile and antioxidative activity of Pericarpium Citri Reticulatae ‘Chachiensis’†
Hong Wanga,
Gu Chen*a,
Xiong Fua and
Rui-Hai Liu*b
aSchool of Food Science and Engineering, South China University of Technology, 381 Wushan Road, 510641, Guangzhou, China. E-mail: chengu@scut.edu.cn; Fax: +86-20-87113849; Tel: +86-13660887090
bDepartment of Food Science, Cornell University, Ithaca, New York 14853, USA. E-mail: rl23@cornell.edu; Fax: +1 607 254 4868; Tel: +1 607 255 6235
First published on 21st October 2016
Abstract
The dried and aged pericarps of Citri Reticulatae are popular edible and medicinal products in southeast and eastern Asia. To investigate the effects of aging on Pericarpium Citri Reticulatae ‘Chachiensis’ (PCR-Chachiensis), free and bound phenolic compounds from representative PCR-Chachiensis were extracted and studied. During aging, bound phenolics and flavonoids were dramatically elevated and their antioxidant activity increased, as revealed by different antioxidative analysis methods. The bound fraction intensified the contribution to the total benefit of aged PCR-Chachiensis, which exhibited higher phenolic and flavonoid content, and superior antioxidant activity. Based on the phenolic composition profile, consisting of eleven characteristic phenolics, multivariate data analysis distinguished samples and attributed the special qualities of aged PCR-Chachiensis to the accumulation of bound phenolic compounds, a fraction previously ignored in research, but typically consumed as a food ingredient. The accumulation of bound phenolic compounds is of great interest for citrus peel utilization as nutraceutical and functional food products.
Introduction
As one of the world's major fruit crops, with global production of more than 100 million metric tons each year, citrus flesh is usually consumed directly or made into juice, while the peels are discarded, which results in about 8 to 20 million tons being disposed, causing a serious environmental problem and significant waste.1,2 Our recent research revealed that regardless of the different ripening stages, higher contents of phenolics and flavonoids, stronger antioxidant and antiproliferative activities were found in the peels than in the flesh of Citrus reticulata Blanco cv. Chachiensis,3 thus implying the great potential for citrus peel utilization. People in southeast and eastern Asia have utilized the dried and aged peels (pericarps) of Citrus reticulata Blanco and its cultivars as Pericarpium Citri Reticulatae (in Chinese Chen Pi) for hundreds of years. As an edible and medicinal ingredient, Pericarpium Citri Reticulatae is widely used in cuisine, such as soup and congee, in tea with Puer or Oolong, and in miscellaneous snacks. It is also used as a traditional medicine to remedy indigestion and respiratory disease. Recent studies explored its diversified functions, such as antioxidant, anticancer, anti-inflammatory, antimicrobial, and antiviral activities, as well as neuroprotective effects, which are mainly attributed to the bioactive volatile oil and/or flavonoids.4–10Originating from the pericarps of Citri Reticulatae Blanco cv. Chachiensis, Pericarpium Citri Reticulatae Chachiensis, hereafter PCR-Chachiensis (in Chinese Guangchenpi), is listed as a premium Pericarpium Citri Reticulatae in the Chinese Pharmacopoeia, due to its outstanding medicinal and health benefits. In the past decade, the production of PCR-Chachiensis increased quickly and is now at over 300 tons per year. Empirically, when PCR-Chachiensis is stored and aged for a longer period, it has a better smell and taste, and features greater beneficial effects, as in the proverb “older is better”. However, the effects of aging on the quality and functionality of PCR-Chachiensis has not been clearly characterised so far. Numerous studies have reported the phytochemical profiles of citrus flesh, but only a few investigations were performed in the pericarps, mainly focusing on free phenolics in the solvent-soluble fraction.11–16 Limited studies investigated the bound phenolics in Pericarpium Citri Reticulatae, and to our knowledge, there have been no such studies carried out on PCR-Chachiensis.
Thus, in the present study, we have investigated the effects of preparation and aging on the phytochemical profile and antioxidant activities of the free and bound phenolic fractions of PCR-Chachiensis. Results indicate that the accumulated bioactive compounds in the bound fraction are of great interest for citrus peel utilization as nutraceutical and functional food products.
Experimental
Chemicals and reagents
Most chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA). Williams' Medium E (WME), insulin, and other cell culture reagents were purchased from Gibco (Grand Island, NY, USA).Sample preparation
Four kinds of PCR-Chachiensis samples were obtained from the same orchard in Xinhui District, Guangdong Province, China (around latitude N22°28′23.91′′; longitude E113°02′41.84′′) and authenticated by Yonghe Chen, Xiangyi Chenpi Company. FRESH and PCR01 were pericarps collected in 2013; FRESH was fresh pericarp and the PCR01 was pericarp that was air dried at room temperature using a continuous flow dryer for 24 h. PCR06 and PCR13 were the aged PCR-Chachiensis, naturally stored in jute bags at room temperature, in the conventional aging process, for five and twelve years, respectively. The moisture content was determined by the oven-drying method, as described previously.3 Briefly, the samples were dried in the oven at 105 °C until constant weight was reached; data are shown in ESI Table 1.† The samples used were mixed from different batches of Pericarpium Citri Reticulatae ‘Chachiensis’ to ensure the reliability. To follow the variation of phytochemicals during the first two years of aging, PCR01 sample was naturally aged in the jute bags at room temperature, in the conventional aging process, for two years and analyzed in 2015.Phenolics extraction
Free phenolics were extracted as previously reported.17 The PCR-Chachiensis sample was ground into powder and blended with cold 80% acetone (2 g: 30 mL) for 5 minutes. The mixture was homogenised on ice with a high-speed blender at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 minutes. After centrifuging at 3000 rpm for 5 min, the supernatant was collected, and the residue was extracted again with 30 mL of chilled 80% acetone and re-extracted thrice. The supernatants were pooled and evaporated at 45 °C to less than 10% of the initial volume. The free phenolics fractions were brought up to 10 mL in deionised water. Each sample was extracted in triplicate and the extracts were stored at −40 °C until analysis.
000 rpm for 3 minutes. After centrifuging at 3000 rpm for 5 min, the supernatant was collected, and the residue was extracted again with 30 mL of chilled 80% acetone and re-extracted thrice. The supernatants were pooled and evaporated at 45 °C to less than 10% of the initial volume. The free phenolics fractions were brought up to 10 mL in deionised water. Each sample was extracted in triplicate and the extracts were stored at −40 °C until analysis.
Bound phenolics were extracted as explained before.17 The residue from the free phenolics extraction was digested with 10 mL of 4 M sodium hydroxide at room temperature for 1 h with shaking. The mixture was acidified to pH 2 by using concentrated hydrochloric acid and then extracted at least six times with ethyl acetate, until the supernatants became colourless. The bound phenolics fractions were obtained after the ethyl acetate evaporated, and the fractions were reconstituted in 10 mL of deionized water. Twenty grams of fresh pericarp were extracted using the same method. The extractions were performed in triplicate and maintained at −40 °C until the analysis.
Determination of the phenolic and flavonoid content
The phenolic content was determined using the Folin–Ciocalteu method with a slight modification, as described previously.3,17 The results were calculated by comparing with the standard curve of gallic acid, and expressed as mg of gallic acid equivalents (GAE)/100 g sample (dry weight, DW). The flavonoid content of a sample was analysed using the previously described sodium borohydride/chloranil colourimetric method.3,17 The results were calculated based on the standard curve of fresh catechin and expressed as mg of catechin equivalents (equiv.)/100 g sample (DW).Determination of antioxidant activity using the peroxyl radical scavenging capacity (PSC) assay, oxygen radical absorbance capacity (ORAC) assay and cellular antioxidant activity (CAA) analysis
The PSC assay was assessed as described previously.18 Briefly, 100 μL of appropriate concentration of samples or controls diluted by 75 mmol L−1 phosphate buffer (pH 7.4) were mixed with 100 μL of 13.26 μmol L−1 dichlorofluorescin diacetate (DCFH-DA) and 50 μL of 40 mmol L−1 2,2′-azobis-amidinopropane (ABAP). The reaction was performed at 37 °C for 20 cycles every 2 min at excitation of 485 nm and emission of 535 nm, using the FilterMax F5 Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). The results were calculated as mol vitamin C equiv. g per sample (DW).The ORAC assay was performed as described previously.19 In brief, 20 μL of the appropriate concentration of the extract, or Trolox standard diluted by 75 mmol L−1 phosphate buffer (pH 7.4), were mixed with 200 μL of 0.96 μmol L−1 fluorescein, then incubated for at least 20 min at 37 °C. After the addition of 20 μL of 119.4 mmol L−1 ABAP, the fluorescence intensity was measured every 4.5 min for 35 cycles at excitation of 485 nm and emission of 535 nm, by the FilterMax F5 Multi-Mode Microplate Reader. The results were calculated by comparing with the standard curve of Trolox, and expressed as μmol Trolox equiv. g per sample (DW).
The CAA assay was performed as described previously with HepG2 cells.20 The CAA unit was calculated from the integrated area under the DCF fluorescence versus time curve, and the EC50 values were calculated from the median effect plot of log(CAA unit/(1 − CAA unit)) versus log(dose). The CAA values were calculated by dividing the EC50 of the quercetin standard by the EC50 of the extracts, which were expressed as μmol quercetin equivalents (QE)/per gram of dry weight.
Phenolic composition analysis using HPLC-PAD
The phenolic content of the free and bound phenolics extracts was qualitatively and quantitatively analysed using a Waters series HPLC system equipped with a binary pump (model 1525), a micro degasser, an autosampler (model 2707), a thermostatically controlled column apartment (model 1500) and a photodiode array detector (model 2998), in accordance with the previous study,21 with a slight modification. The samples were separated at 25 °C in a Waters Sun Fire™ C18 column (5 μm, 4.6 × 250 mm). The mobile phase was composed of 0.1% trifluoroacetic acid (aqueous) (A) and acetonitrile (B). Chromatographic peaks were identified by comparing the retention times in specific UV spectra with authentic standards.Statistical analysis
The data are presented as the mean ± standard deviation (SD) from at least three replicates per analysis. The results were analysed among groups using one-way analysis of variance (ANOVA) and Ducan's multiple comparison post-test. A p value less than 0.05 was considered statistically significant. Correlation coefficients were calculated using Pearson's correlation and SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Principal component analysis (PCA) and partial least squares-discriminant analysis (PLS-DA) were performed by SIMCA-P+ V11.5 (Umetrics AB, Umea, Sweden).Results and discussion
Total phenolic and flavonoid content during the preparation of aged PCR-Chachiensis
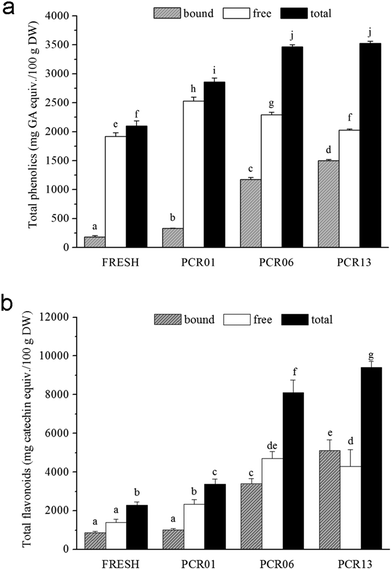
PCR-Chachiensis samples from four representative stages were selected. Fresh and newly dried Citri Reticulatae ‘Chachiensis’ pericarp samples (FRESH and PCR01), as well as PCR-Chachiensis aged for 5 and 12 years (PCR06 and PCR13), were collected to represent different stages of preparation of aged PCR-Chachiensis. As shown in ESI Table 1,† the outside flavedo colour transformed from orange to dark green, then to brown, and finally, to dark brown during the drying and aging process. The inside albedo colour gradually changed from white to light brown. The moisture content decreased significantly from 81.11 ± 1.81% to 18.69 ± 0.10% after the first sunlight and then room temperature drying. It exhibited only a slight decrease to 13.37 ± 0.21% during the first five years of aging and was relatively constant thereafter.Free and bound phenolic compounds were extracted as described in the Experimental section. Expressed as milligrams of gallic acid equivalent per 100 gram of sample on a dry weight basis, the bound, free and total phenolic contents showed notable variations during preparation (Fig. 1a). The bound phenolic content increased from 180.38 ± 26.15 in FRESH to 328.96 ± 7.45 mg GA equiv./100 g DW in PCR01, and increased during aging to 1175.94 ± 35.56 and 1499.76 ± 18.31 mg GA equiv./100 g DW in PCR06 and PCR13, respectively. The free phenolic content also increased from 1918.95 ± 62.34 to 2527.75 ± 68.88 mg GA equiv./100 g DW after drying, but gradually decreased during aging to 2291.07 ± 44.60 and 2026.66 ± 21.51 mg GA equiv./100 g DW in PCR06 and PCR13, respectively. The contribution of bound phenolics to the total phenolics increased during preparation from 8.59% (FRESH) to 11.51% (PCR01), 33.92% (PCR06), and 42.53% (PCR13). Thus, the total phenolic content increased from 2099.33 ± 87.34 to 2856.71 ± 69.43 mg GA equiv./100 g DW after drying, and accumulated during the first five years of aging to approximately 3467.01 mg GA equiv./100 g DW in PCR06. However, it remained relatively constant thereafter.
The free, bound and total flavonoid content of the samples, expressed as milligrams of catechin equivalent per 100 gram dry weight, showed interesting variations (Fig. 1b). The bound flavonoid content increased from 880.58 ± 63.24 to 1024.50 ± 53.52 mg catechin equiv./100 g DW after drying, but this increase was not significant, compared with the dramatic accumulation of bound flavonoid during aging. The bound flavonoid tripled, at 3396.09 ± 281.38 mg catechin equiv./100 g DW in PCR06 and was even higher, 5115.27 ± 565.03 mg catechin equiv./100 g DW, in PCR13. The free flavonoid content increased gradually during drying and the first five years of aging from 1410.45 ± 167.19 in FRESH to 2344.68 ± 231.64 and 4712.65 ± 357.67 mg catechin equiv./100 g DW in PCR01 and PCR06, respectively. It remained relatively constant thereafter. The contribution of bound flavonoids to the total flavonoids decreased after drying from 38.44% (FRESH) to 30.41% (PCR01), but increased during aging to 41.88% and 54.31% in PCR06 and PCR13, respectively. The total flavonoid content increased during the drying and aging processes from 2291.03 ± 177.66 in FRESH, 3369.18 ± 284.54 in PCR01 to 8108.75 ± 638.11 in PCR06 and 9418.58 ± 296.73 mg catechin equiv./100 g DW in PCR13.
We measured the phenolic and flavonoid content in both the free and bound fraction, and observed a notable increase in the bound fraction during aging (Fig. 1). Choi et al. compared the dried Citrus peels (Pericarpium Citri Reticulatae, Chenpi) stored for 1 and 3 years, and reported an increase of free phenolics in the 3 year samples, while the insoluble-bound phenolic fraction was relatively constant.16 Such differences from our observations might be due to the different citrus varieties, processing conditions and aging periods. Bound phenolics in plant-derived foods are interesting sources of phenolics and have garnered much attention recently, such as in reports on Adinandra tea,17 grape and pomegranate wine,22 dehulled highland barley,23 spent coffee,24 lentils during germination,25 rice bran,26 soy,27 and maize.28 The minimal release of bound phenolics after simulated gastric and small intestinal digestion indicates that polyphenols bound to the plant cell wall might be transported to the colon and likely released there by cell wall-degrading bacteria.29 This scenario suggests the potential health benefits of bound phenolics in plant-derived foods. In our study, we found that the bound phenolics and flavonoids accumulated in the aged PCR-Chachiensis might be released in the colon and are related to the traditional medicinal application of treating indigestion.
Variations in antioxidant activity during aged PCR-Chachiensis preparation
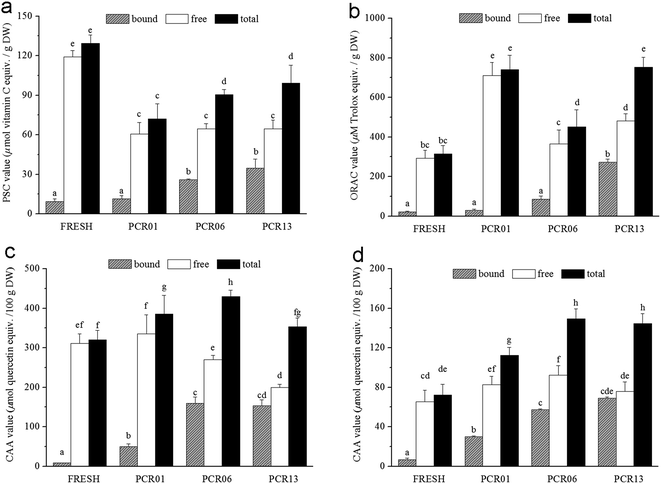
The in vitro antioxidant activities were first evaluated using PSC and ORAC assays. Expressed as micromoles of vitamin C equivalent per gram of sample, the PSC values showed interesting variations (Fig. 2a). The PSC values for bound fractions from FRESH (9.41 ± 2.02) and PCR01 (11.5 ± 2.45) were comparable, while it increased during aging to 25.89 ± 0.72 in PCR06 and 34.75 ± 6.97 in PCR13; however, the increase in PCR13 was not significant, compared with PCR06. The PSC value of the free phenolic fraction decreased significantly after drying from 119.03 ± 4.78 in FRESH to 60.56 ± 8.92 in PCR01, and it was relatively constant during aging. The PSC value of the total fraction decreased after drying and increased by approximately 25.6% during the first year of aging, but the enhancement was not significant thereafter.The ORAC value is expressed as micromoles of Trolox equivalents per gram of sample and is shown in Fig. 2b. Similar to the PSC assay results, the ORAC values for the FRESH (22.06 ± 2.53) and PCR01 (29.11 ± 7.13) bound fractions were comparable, and they increased during aging to 86.69 ± 14.85 in PCR06 and 271.89 ± 16.7 in PCR13, which is approximately a ten-fold enhancement, compared with PCR01. The ORAC value of the free fraction exhibited a different trend compared with the PSC value. It increased significantly after drying from 293.02 ± 40.12 in FRESH to 711.72 ± 65.36 in PCR01, and then it declined dramatically to 364.5 ± 71.43 in PCR06 and increased to 481.64 ± 35.84 in PCR13. The ORAC value of the total fraction doubled after drying, from 315.08 ± 42.19 in FRESH to 740.83 ± 72.35 in PCR01, and then it decreased to 451.18 ± 85.96 in PCR06 and increased to 753.52 ± 50.29 in PCR13, which was at a similar level to PCR01.
To better mimic in vivo conditions, different cellular models were usually applied to analyse the antioxidant activity of polyphenol extracts.20,30,31 Here, the cellular antioxidant activity was further evaluated in HepG2 cells using the CAA assay. The CAA value was calculated based on comparison of the sample and quercetin EC50 values, and was expressed as micromoles of quercetin equivalence per 100 gram of sample, as shown in Fig. 2. Generally, the CAA value in the no PBS wash protocol (Fig. 2c) was higher than in the PBS wash protocol (Fig. 2d) because only the antioxidants that were absorbed into the cells or strongly associated with the cellular membrane were evaluated in the PBS wash protocol. The bound fraction CAA values showed a similar trend in both protocols (Fig. 2c and d). In the no-PBS-wash protocol, it increased after drying and in the first five years of aging from 8.77 ± 0.39 in FRESH, to 50.13 ± 7.22 in PCR01 and 159.66 ± 15.66 in PCR06. The comparable CAA values in PCR06 and PCR13 indicated that it was relatively constant during the later aging process. In the PBS wash protocol, the bound fraction CAA value increased by approximately 5 fold after drying and approximately doubled during the first five years of aging, and it was relatively constant thereafter. The CAA value trends for the free fraction differed between the no-PBS-wash and PBS wash protocols. In the no-PBS-wash protocol, the value remained steady after drying and decreased during aging from 335.61 ± 47.48 in PCR01 to 270.32 ± 10.88 and 199.85 ± 8.16 in PRC06 and PCR13, respectively. In the PBS wash protocol, the CAA value variation in the free fraction was subtle. The CAA values for the total fraction combining the contributions from the free and bound fractions increased after drying and during aging for the first five years in both the no-PBS-wash and PBS wash protocols; in later aging processes, they decreased in the no-PBS-wash protocol, but remained steady in the PBS wash protocol.
The different antioxidant activity assays PSC, ORAC and CAA were used to evaluate the peroxyl radical scavenging capacity, oxygen radical absorbance capacity and cellular antioxidant activity, respectively.18–20 Regardless of the assays used, in vitro or ex vivo, the PSC or ORAC assay, the bound fraction showed increasing antioxidant activity during aging and presented an increased contribution to the total antioxidant activities. The contribution of the bound fraction to the PSC value increased during aging from 15.96% in PCR01 and 28.59% in PCR06 to 35% in PCR13. The contribution of the bound fraction to the ORAC value increased during aging from 3.93% in PCR01 and 19.21% in PCR06 to 36.08% in PCR13. Impressively, for the CAA value, the contribution of the bound fraction also increased during aging from 13% in PCR01 and 37.13% in PCR06 to 43.38% in PCR13 for the no-PBS-wash protocol, as well as from 26.55% in PCR01 and 38.29% in PCR06 to 47.62% in PCR13 for the PBS wash protocol. These results are consistent with the increased contribution of the bound phenolics and flavonoids to the total phenolics and flavonoids.
Phenolic compound compositions during the preparation of PCR-Chachiensis
The phenolic compositions of the free and bound fractions from different PCR-Chachiensis samples were analysed by HPLC (Fig. 3) and eleven phenolic acids and flavonoids were identified and quantified, as presented in Table 1.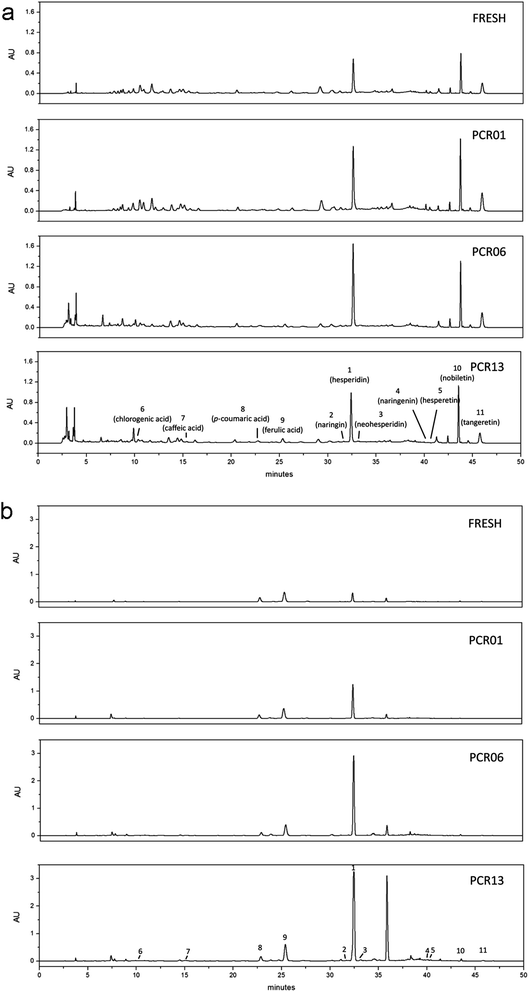 | ||
| Fig. 3 HPLC profiles (0–50 min) at 283 nm for the free (a) and bound (b) fractions of PCR-Chachiensis samples. | ||
| Sample | Phenolic acid | Flavonoid | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorogenic acid | Caffeic acid | p-Coumaric acid | Ferulic acid | Naringin | Hesperidin | Neohesperidin | Naringenin | Hesperetin | Nobiletin | Tangeretin | ||
| a Values (μg g−1 DW) in the same column with different letters indicate significant differences at p < 0.05. ND: not detected. | ||||||||||||
| FRESH | Bound | 20.72 ± 0.03a | 23.32 ± 0.01a | 21.39 ± 0.02a | 498.99 ± 3.27e | 23.90 ± 0.70a | 484.38 ± 25.80a | 18.80 ± 0.67a | 8.90 ± 0.67a | 10.53 ± 0.69a | 50.66 ± 0.15a | 17.01 ± 0.01a |
| Free | 519.14 ± 14.90f | 223.08 ± 4.46e | 152.77 ± 2.57c | 356.09 ± 2.26b | 581.38 ± 49.53ef | 7497.15 ± 289.77f | 288.19 ± 47.42b | 48.46 ± 1.22d | 129.68 ± 5.83c | 1695.06 ± 78.68b | 645.73 ± 33.64c | |
| Total | 539.86 ± 14.88g | 246.41 ± 4.45g | 174.15 ± 2.56de | 855.09 ± 5.22i | 605.28 ± 50.09f | 7981.53 ± 315.55g | 307.00 ± 47.46bc | 57.36 ± 0.57e | 140.21 ± 5.21d | 1745.72 ± 78.71b | 662.74 ± 33.65c | |
| PCR01 | Bound | 47.53 ± 0.41b | 54.16 ± 0.14b | ND | 350.97 ± 2.76ab | 39.71 ± 0.04a | 1022.94 ± 27.55b | 38.87 ± 0.13a | 20.02 ± 0.01b | 19.80 ± 0.05b | 57.34 ± 1.69a | 23.82 ± 0.39a |
| Free | 730.06 ± 16.13h | 294.47 ± 5.45h | 187.50 ± 3.90f | 409.14 ± 0.99c | 513.47 ± 2.17d | 6731.10 ± 182.80e | 373.05 ± 48.98cd | 60.94 ± 0.53g | 179.78 ± 1.90f | 2602.22 ± 43.85e | 908.24 ± 19.37d | |
| Total | 777.59 ± 16.48i | 348.63 ± 5.59i | 187.50 ± 3.90f | 760.11 ± 2.99g | 553.18 ± 2.17de | 7754.04 ± 164.08fg | 411.92 ± 49.09d | 80.96 ± 0.52i | 199.58 ± 1.94g | 2659.55 ± 45.48e | 932.06 ± 19.60d | |
| PCR06 | Bound | 44.54 ± 0.15b | 51.08 ± 0.03b | 45.37 ± 0.21b | 346.20 ± 2.47a | 53.31 ± 0.93a | 2319.24 ± 68.60c | 39.54 ± 2.59a | 19.21 ± 0.70b | 20.06 ± 1.22b | 61.95 ± 0.18a | 24.40 ± 0.05a |
| Free | 328.29 ± 13.92d | 194.77 ± 2.27d | 178.41 ± 2.21e | 492.84 ± 3.41e | 290.69 ± 20.81b | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 523.48 ± 235.90h 523.48 ± 235.90h |
294.44 ± 56.65bc | 58.40 ± 1.17ef | 140.93 ± 4.11d | 2163.21 ± 35.36d | 655.28 ± 49.57c | |
| Total | 372.83 ± 13.82e | 245.85 ± 2.28g | 223.78 ± 2.27h | 839.04 ± 2.27h | 330.87 ± 22.49bc | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 842.73 ± 208.36i 842.73 ± 208.36i |
333.98 ± 54.87bc | 77.61 ± 1.00h | 161.00 ± 4.56e | 2225.16 ± 35.50d | 679.68 ± 49.62c | |
| PCR13 | Bound | 45.12 ± 0.01b | 52.03 ± 0.05b | 45.63 ± 0.03b | 465.71 ± 6.74d | 53.31 ± 0.93a | 3444.44 ± 259.46d | 58.06 ± 4.99a | 23.35 ± 0.42c | 23.96 ± 0.24b | 80.70 ± 0.66a | 30.95 ± 0.14a |
| Free | 275.78 ± 9.05c | 181.81 ± 1.05c | 169.58 ± 6.51d | 555.67 ± 6.82f | 294.96 ± 39.26b | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 897.36 ± 307.59h 897.36 ± 307.59h |
253.03 ± 59.38b | 59.33 ± 1.24f | 139.13 ± 6.38d | 2054.49 ± 106.47c | 555.83 ± 46.63b | |
| Total | 320.90 ± 9.06d | 233.84 ± 1.05f | 215.2 ± 6.51g | 1021.38 ± 10.87j | 348.26 ± 40.19c | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 341.8 ± 439.28j 341.8 ± 439.28j |
311.09 ± 59.07bc | 82.68 ± 1.60j | 163.09 ± 6.23e | 2135.19 ± 106.45cd | 586.78 ± 46.59b | |
Among the four phenolic acids analysed, the most abundant one was ferulic acid, followed by chlorogenic acid then caffeic acid and p-coumaric acid. In contrast to the remaining three phenolic acids, which were contributed mainly by the free phenolic fraction (77.75% to 96.16%), a large portion of the ferulic acid was from the bound phenolic fraction, ranging from 41.26% to 58.36%. The bound ferulic acid decreased from 498.99 ± 3.27 μg g−1 DW in FRESH to 350.97 ± 2.76 μg g−1 DW in PCR01 after drying, and was steady during the first five years of aging, but increased dramatically during the later aging process to 465.71 ± 6.74 μg g−1 DW in PCR13. The ferulic acid in the free phenolic fraction increased during the preparation from 356.09 ± 2.26 μg g−1 DW in FRESH, to 409.14 ± 0.99 and 492.84 ± 3.41 μg g−1 DW in PCR01 and PCR06, respectively, and 555.67 ± 6.82 μg g−1 DW in PCR13. Considering the contributions from both the free and bound fractions, although the samples exhibited a slight decrease after drying, the total ferulic acid significantly increased during aging by approximately 10.38% and 21.73% during the first five years and the later aging process, respectively. The bound chlorogenic acid and caffeic acid increased after drying and remained relatively steady during aging. The free chlorogenic acid and caffeic acid increased after drying but decreased during the aging process. When comparing the phenolic acid composition between the Pericarpium Citri Reticulatae stored for 1 and 3 years, Choi et al. reported a notable increase in free ferulic acid, which is consistent with our observations.16 However, the free caffeic acid notably increased and the bound p-coumaric acid significantly decreased in the Pericarpium Citri Reticulatae stored for 3 years.16 Such differences might be due to the different varieties, as well as aging conditions and terms used.
Among the seven flavonoids analysed, the most abundant one was hesperidin, which is the characteristic flavonoid in Pericarpium Citri Reticulatae, followed by two representative polymethoxylated flavones, nobiletin and tangeretin (Table 1). During preparation of the aged PCR-Chachiensis, the most impressive increase in flavonoids was observed in the bound hesperidin, which contributed to the remarkable increase in bound phenolics and flavonoids observed in Fig. 1. It increased from 484.38 ± 25.80 μg g−1 DW in FRESH to 1022.94 ± 27.55, 2319.24 ± 68.60, and 3444.44 ± 259.46 μg g−1 DW in PCR01, PCR06 and PCR13, respectively. In contrast to the greater than one-fold increase in bound hesperidin, we observed an approximate 9.56% decrease in the free hesperidin after drying; however, the total hesperidin did not differ significantly between FRESH and PCR01. During the first five years of aging, we observed an approximate 126.72% and 71.20% increase in the free and bound hesperidin, which yielded an approximate 78.52% increase in the total hesperidin. During the later aging process, the increase in free hesperidin was not significant, while the bound hesperidin increased to 48.52%, which contributed to the notable increase in total hesperidin. The contribution of the bound hesperidin to the total hesperidin increased from 6.07% in FRESH to 13.20%, 16.75% and 22.45% in PCR01, PCR06 and PCR13, respectively. Similarly, the bound contribution for the remaining flavonoids, such as naringin, naringenin, neohesperidin and hesperetin, also increased during the preparation. Certain increases were due to the increase in the bound fraction, and the remaining increases were attributed to the decrease in the free fraction. Compared with the other flavonoids analysed, the percentage of bound nobiletin and tangeretin to the total fraction was small, ranging from 2.16% to 3.78% for nobiletin and 2.56% to 5.27% for tangeretin. The increase in bound nobiletin and tangeretin was not significant during the preparation. The changing trend for the total nobiletin and tangeretin was mainly determined by the free fraction (accounting for 94.73–97.84%), which increased after drying, but decreased during the aging process.
Multivariate statistical analysis of PCR-Chachiensis during preparation
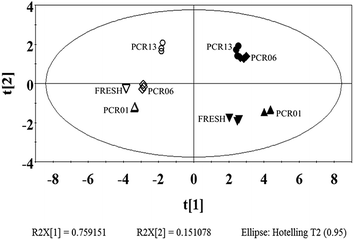
To map the PCR-Chachiensis samples at different preparation stages and understand the basic principles contributing to the differences, multivariate data analysis was employed to analyse the phenolic composition and content.Fig. 4 shows the principal component analysis (PCA) score plot based on the first two principal components (PC1 and PC2) that explained the top two greatest total variance percentages. The PC1 and PC2 explained 75.92% and 15.11% of the sample diversity, respectively. The bound and free fraction samples are clearly separated on the left and right regions of the score plot. Most of the samples from different preparation stages are clearly distinguished. However, the free fraction for PCR06 and PCR13 are close, which indicates a similarity in phenolic profiles and a subtle change in the free phenolics during the later aging process. In contrast, the bound fraction for PCR13 is distal to PCR06 and the other bound fractions, which indicates a notable change in the bound phenolics during the later aging process, and their contribution to the special qualities of aged PCR-Chachiensis. Examining the variable loading shows their contribution to the first two PCs. As shown in ESI Table 2,† PC1 is essentially a function of caffeic acid, neohesperidin, naringenin, hesperetin, nobiletin and tangeretin; PC2 is associated with ferulic acid and the total flavonoids. Similar results were obtained in the partial least squares-discriminant analysis (PLS-DA) (ESI Fig. 1†). Thus, it was implied that the key phenolics that contribute to the special qualities of aged PCR-Chachiensis might exist in the bound fraction, which was not well characterised previously, and deserves further investigation.
Variation of total phenolic and flavonoid content during two years' aging of PCR-Chachiensis
Since the phenolic compositions are variable, dependent on soil, climate conditions as well as aging conditions, etc., we further followed the phenolic variation of the same batch of samples for two years. Increases in bound phenolics and flavonoids were obvious in PCR01, while significant variation was found in the bound fraction (Table 2). The bound phenolics and flavonoids increased more than three fold in PCR01, and the characteristic flavonoid, hesperidin, increased to 9615.30 ± 83.83 and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 481.34 ± 61.47 μg g−1 DW in the bound and free extract of PCR01, respectively. Such dramatic increases implied the active transformation of phenolics during aging and it might involve enzymatic and non-enzymatic reactions, which deserve further investigation.
481.34 ± 61.47 μg g−1 DW in the bound and free extract of PCR01, respectively. Such dramatic increases implied the active transformation of phenolics during aging and it might involve enzymatic and non-enzymatic reactions, which deserve further investigation.
| PCR01 analyzed in 2013 | PCR01 analyzed in 2015 | |||||
|---|---|---|---|---|---|---|
| Bound | Free | Total | Bound | Free | Total | |
| a Values are expressed in mg GAE/100 g DW for total phenolics, mg CE/100 g DW for total flavonoids and μg g−1 DW for phenolic composition. | ||||||
| Total phenolics | 328.96 ± 7.45 | 2527.75 ± 68.88 | 2856.71 ± 69.43 | 1133.22 ± 31.75 | 2640.04 ± 63.14 | 3773.26 ± 78.60 |
| Total flavonoids | 1024.05 ± 53.52 | 2344.68 ± 231.64 | 3369.18 ± 284.54 | 3463.24 ± 55.40 | 7712.00 ± 700.86 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 175.24 ± 735.29 175.24 ± 735.29 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Phenolic acid | ||||||
| Chlorogenic acid | 47.53 ± 0.41 | 730.06 ± 16.13 | 777.59 ± 16.48 | 25.78 ± 0.49 | 155.83 ± 3.33 | 181.61 ± 3.37 |
| Caffeic acid | 54.16 ± 0.14 | 294.47 ± 5.45 | 348.63 ± 5.59 | 43.03 ± 0.58 | 438.80 ± 2.34 | 481.83 ± 2.92 |
| p-Coumaric acid | ND | 187.50 ± 3.90 | 187.50 ± 3.90 | 204.17 ± 1.10 | 202.82 ± 0.47 | 407.00 ± 1.02 |
| Ferulic acid | 350.97 ± 2.76 | 409.14 ± 0.99 | 760.11 ± 2.99 | 975.48 ± 1.61 | 517.65 ± 1.67 | 1493.13 ± 3.25 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Flavonoid | ||||||
| Naringin | 39.71 ± 0.04 | 513.47 ± 2.17 | 553.18 ± 2.17 | 59.75 ± 1.22 | 393.05 ± 23.77 | 452.80 ± 22.62 |
| Hesperidin | 1022.94 ± 27.55 | 6731.10 ± 182.80 | 7754.04 ± 164.08 | 9615.30 ± 83.83 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 481.34 ± 61.47 481.34 ± 61.47 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 096.64 ± 79.00 096.64 ± 79.00 |
| Neohesperidin | 38.87 ± 0.13 | 373.05 ± 48.98 | 411.92 ± 49.09 | 85.63 ± 1.38 | 315.71 ± 18.4 | 401.34 ± 17.04 |
| Naringenin | 20.02 ± 0.01 | 60.94 ± 0.53 | 80.96 ± 0.52 | 30.01 ± 0.61 | 137.99 ± 4.52 | 168.00 ± 3.95 |
| Hesperetin | 19.80 ± 0.05 | 179.78 ± 1.90 | 199.58 ± 1.94 | 49.66 ± 2.00 | 132.04 ± 6.26 | 181.70 ± 7.36 |
| Nobiletin | 57.34 ± 1.69 | 2602.22 ± 43.85 | 2659.55 ± 45.48 | 85.63 ± 1.38 | 2553.87 ± 11.37 | 2639.5 ± 12.49 |
| Tangeretin | 23.82 ± 0.39 | 908.24 ± 19.37 | 932.06 ± 19.60 | 31.50 ± 1.56 | 1000.86 ± 2.80 | 1032.36 ± 3.79 |
The increase in phenolics, flavonoids and hesperidin from the bound fraction during aging might contribute to the special qualities of aged PCR-Chachiensis, supporting the old proverb for PCR-Chachiensis, “older is better”. An increase in phenolic content was reported for sun-dried dates (Phoenix dactylifera L.), compared to fresh dates; it was suggested that the tannin degradation by temperature and maturation enzymes might lead to phenolic compound accumulation.32 However, the increase in the phenolic and flavonoid content of PCR-Chachiensis during such a long aging process was apparently more complex than the tannin degradation. Aging is considered to be the main factor determining the quality and value of Pu'er, a fermented black tea. The polyphenol content of both raw (un-accelerated fermented) and ripened (accelerated fermented) Pu'er increased during the first 60 months of aging and then decreased, which was correlated to the changes in fungal diversity.33 Both fungi and bacteria were suggested to contribute to the conversion of chemical constituents, including polyphenols in fermented tea.34,35 We have identified bacteria and fungi in PCR-Chachiensis through culture-dependent and independent approaches (unpublished data). These microorganisms might contribute to the increases in bound phenolic and flavonoid contents during aging. The underlying mechanism is undergoing exploration and might contribute to citrus peel utilization worldwide.
Conclusions
In this work, the results revealed interesting variations in the composition of free and bound phenolic compounds and antioxidative activities during the preparation of aged PCR-Chachiensis. Generally, the free phenolics fraction had higher phenolic and flavonoid contents, more individual phenolics, and stronger antioxidative activities than the bound phenolics fraction. However, the contribution of the bound fraction increased during aging. A dramatic increase was observed during the aging process in the bound phenolics and flavonoid contents, as well as in their antioxidant activity, which was revealed by PSC, ORAC and CAA assays. The multivariate data analysis distinguished the samples during preparation and attributed the special qualities of aged PCR-Chachiensis to the accumulation of bound phenolic compounds, a fraction that was previously ignored in research but is typically consumed as a food ingredient. During the first two years of aging, total phenolics, flavonoids and characteristic flavonoids, such as hesperidin, increased dramatically in the PCR01 sample, especially in the bound fraction, thus implying the active transformation of phenolics during aging, the mechanism of which deserves further investigation. The results here provide valuable information for the comprehensive evaluation of the phenolic composition and bioactivities in aged Pericarpium Citri Reticulatae, and might contribute to the future utilization of citrus peel as nutraceutical and functional food products.Conflict of interest
The authors declare that they have no conflict of interest.Acknowledgements
The study was funded by National Natural Science Foundation of China (Grant No. 31270085), “Leading Talent of Guangdong Province” and the Science and Technology Program of Guangdong Province (Grant No. 2013B090700008).References
- Y. Liu, E. Heying and S. A. Tanumihardjo, Compr. Rev. Food Sci. Food Saf., 2012, 11, 530–545 CrossRef CAS.
- K. Rezzadori, S. Benedetti and E. R. Amante, Food Bioprod. Process., 2012, 90, 606–614 CrossRef CAS.
- H. Wang, G. Chen, X. Guo, A. M. Abbasi and R. Liu, LWT--Food Sci. Technol., 2016, 69, 67–75 CrossRef CAS.
- B. Gao, Y. Chen, M. Zhang, Y. Xu and S. Pan, Molecules, 2011, 16, 4082–4096 CrossRef CAS.
- S.-C. Ho and C.-T. Kuo, Food Chem. Toxicol., 2014, 71, 176–182 CrossRef CAS PubMed.
- S. C. Ho and C. C. Lin, J. Agric. Food Chem., 2008, 56, 7976–7982 CrossRef CAS PubMed.
- S. L. Hwang, P. H. Shih and G. C. Yen, J. Agric. Food Chem., 2012, 60, 877–885 CrossRef CAS PubMed.
- L. Liu, X. Xu, D. Cheng, X. Yao and S. Pan, J. Agric. Food Chem., 2012, 60, 4336–4341 CrossRef CAS PubMed.
- E. Tripoli, M. La Guardia, S. Giammanco, D. Di Majo and M. Giammanco, Food Chem., 2007, 104, 466–479 CrossRef CAS.
- J. J. Xu, X. Wu, M. M. Li, G. Q. Li, Y. T. Yang, H. J. Luo, W. H. Huang, H. Y. Chung, W. C. Ye, G. C. Wang and Y. L. Li, J. Agric. Food Chem., 2014, 62, 2182–2189 CrossRef CAS PubMed.
- G. Zheng, D. Yang, D. Wang, F. Zhou, X. Yang and L. Jiang, J. Agric. Food Chem., 2009, 57, 6552–6557 CrossRef CAS PubMed.
- W. Li, Z. Wang, Y.-P. Wang, C. Jiang, Q. Liu, Y.-s. Sun and Y.-n. Zheng, Food Chem., 2012, 130, 1044–1049 CrossRef CAS.
- E. H. Liu, P. Zhao, L. Duan, G.-D. Zheng, L. Guo, H. Yang and P. Li, Food Chem., 2013, 141, 3977–3983 CrossRef CAS PubMed.
- G.-D. Zheng, P. Zhou, H. Yang, Y.-s. Li, P. Li and E. H. Liu, Food Chem., 2013, 136, 604–611 CrossRef CAS PubMed.
- T. Li, X. Li, M. Zhang, C. Jiang, L. Hu and X. Yang, Food Anal. Method, 2014, 7, 89–99 CrossRef.
- M. Y. Choi, C. Chai, J. H. Park, J. Lim, J. Lee and S. W. Kwon, J. Pharm. Biomed., 2011, 54, 638–645 CrossRef CAS PubMed.
- Y. Chen, G. Chen, X. Fu and R.-h. Liu, J. Agric. Food Chem., 2015, 63, 169–176 CrossRef CAS PubMed.
- K. K. Adom and R. H. Liu, J. Agric. Food Chem., 2005, 53, 6572–6580 CrossRef CAS PubMed.
- D. J. Huang, B. X. Ou, M. Hampsch-Woodill, J. A. Flanagan and R. L. Prior, J. Agric. Food Chem., 2002, 50, 4437–4444 CrossRef CAS PubMed.
- K. L. Wolfe and R. H. Liu, J. Agric. Food Chem., 2007, 55, 8896–8907 CrossRef CAS PubMed.
- M. X. Zhang, C. Q. Duan, Y. Y. Zang, Z. W. Huang and G. J. Liu, Food Chem., 2011, 129, 1530–1536 CrossRef CAS.
- D. Z. Lantzouraki, V. J. Sinanoglou, T. Tsiaka, C. Proestos and P. Zoumpoulakis, RSC Adv., 2015, 5, 101683–101692 RSC.
- Y. Zhu, T. Li, X. Fu, A. M. Abbasi, B. Zheng and R. H. Liu, J. Funct. Foods, 2015, 19, 439–450 CrossRef CAS.
- C. Monente, I. A. Ludwig, A. Irigoyen, M.-P. De Pena and C. Cid, J. Agric. Food Chem., 2015, 63, 4327–4334 CrossRef CAS PubMed.
- J. Yeo and F. Shahidi, J. Agric. Food Chem., 2015, 63, 379–381 CrossRef CAS PubMed.
- W. Wang, J. Guo, J. Zhang, J. Peng, T. Liu and Z. Xin, Food Chem., 2015, 171, 40–49 CrossRef CAS PubMed.
- V. Verardo, Y. Riciputi, A. Garrido-Frenich and M. F. Caboni, Food Chem., 2015, 185, 239–244 CrossRef CAS PubMed.
- A. K. Das and V. Singh, J. Funct. Foods, 2015, 13, 363–374 CrossRef CAS.
- A. Padayachee, G. Netzel, M. Netzel, L. Day, D. Mikkelsen and M. J. Gidley, Food Funct., 2013, 4, 906–916 CAS.
- Y. Y. Bian, J. Guo, K. X. Zhu, X. N. Guo, W. Peng and H. M. Zhou, RSC Adv., 2015, 5, 16116–16124 RSC.
- M. Cruz, P. Antunes, L. Paulo, A. M. Ferreira, A. Cunha, C. Almeida-Aguiar and R. Oliveira, RSC Adv., 2016, 6, 49806–49816 RSC.
- M. Al-Farsi, C. Alasalvar, A. Morris, M. Baron and F. Shahidi, J. Agric. Food Chem., 2005, 53, 7592–7599 CrossRef CAS PubMed.
- J. Q. Tian, Z. X. Zhu, B. Wu, L. Wang and X. Z. Liu, J. Food Sci., 2013, 78, M1249–M1256 CrossRef CAS PubMed.
- J. F. Tan, W. D. Dai, M. L. Lu, H. P. Lv, L. Guo, Y. Zhang, Y. Zhu, Q. H. Peng and Z. Lin, Food Res. Int., 2016, 79, 106–113 CrossRef CAS.
- Q. Wang, J. Gong, Y. Chisti and S. Sirisansaneeyakul, J. Food Sci., 2015, 80, M809–M817 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra22082g |
| This journal is © The Royal Society of Chemistry 2016 |