DOI:
10.1039/C6RA21993D
(Paper)
RSC Adv., 2016,
6, 104754-104762
Synthesis, characterization, and highly acid-resistant properties of crosslinking β-chitosan with polyamines for heavy metal ion adsorption†
Received
2nd September 2016
, Accepted 28th October 2016
First published on 28th October 2016
Abstract
This study primarily used β-chitosan as the main reactant and benzaldehyde as the protecting group to convert the amine groups on location C2 of chitosan into Schiff bases. Grafting and crosslinking of epichlorohydrin and triethylenetetramine with chitosan was conducted and the structural changes were analyzed with Fourier transform infrared spectroscopy (FTIR), UV-visible spectroscopy (UV-Vis), thermogravimetric analysis (TGA), elemental analyses (EA), differential scanning calorimeter (DSC), X-ray powder diffraction (XRD), nuclear magnetic resonance (NMR), and scanning electron microscope (SEM) to prove the successful modification to form crosslinked β-chitosan. Lastly, this study compared the heavy metal ion (such as Cu2+ and Ag+) adsorption capacities of chitosan before and after the graft modification. The capacity of modified chitosan for adsorbing copper ions improved in acid environments with pH of 2 to 6. The capacity increased from 67.76 mg g−1 to 117.60 mg g−1 when the pH value was 6. In addition, the capacity of adsorbing silver ions was higher than that of adsorbing copper ions and it enhanced from 116.80 mg g−1 to 151.20 mg g−1 when the pH level was 6. Furthermore, the modified crosslinked β-chitosan had an excellent acid-resistant property.
1. Introduction
With the development of global industrial production, the heavy metal pollution of water resources has become an environmental problem in urgent need of a solution among the numerous environmental issues.1,2 The heavy metal ion pollution mainly results from various industrial production.3,4 Heavy metals, including, lead (Pb),5 cadmium (Cd),6 zinc (Zn),7 mercury (Hg),8 arsenic (As),9 silver (Ag),10 chromium (Cr),11 copper (Cu),12 iron (Fe),13 etc. are the main industrial pollutants. For example, an important issue to be resolved for the electroplating and washing wastewater in the electroplating industry, the Cu and Ag dust pollution caused by metal smelting and post processing in the processing industry of nonferrous metals.14–16 Pollution is caused by the pollutants' entering the surface and underground water by different ways, which damages the ecological environment and threatens human health. Kwon et al. studied the heavy metal pollution in grains caused by mining in South Korea and showed that such pollution can lead to heavy metal deposition in the bodies of local residents.17 Saha et al. analyzed the fish and shellfish in the Bay of Bengal and found that local residents who eat fish and shellfish polluted by heavy metals have a higher risk of getting cancer.18 Liu et al. analyzed leafy and root vegetables in Hangzhou and Changxing in Zhejiang Province and found that the leafy and root vegetables polluted by heavy metals are more dangerous than the polluted solanaceous and legume vegetables.19 Consequently, they suggested that the local residents should refrain from eating a lot of such vegetables to avoid heavy metal accumulation in their bodies.
Currently, there are many approaches to remove heavy metal wastewater from industrial wastewater to reduce environmental hazard and prevent pollution. The heavy metal wastewater treatment methods can be classified into three categories. (1) Chemical treatment: it removes heavy metal ions by chemical reactions, including precipitation and electrochemical reduction.20–22 The precipitation method can remove sulfide and ferrite by neutralization reactions, but the added neutralization precipitants are very likely to cause secondary pollution and the processing effect is limited by water quality, thereby, restricting the engineering application of this method. (2) Physical treatment: it includes physical adsorption, solvent extraction, ion exchange, and membrane separation (separating the membranes without changing the chemical forms of heavy metal ions); each of these has its own advantages and disadvantages.23–25 Taking physical adsorption as an example, it has a disadvantage that the raw materials have a weak heavy metal adsorption capacity, which needs to be improved by chemical modification. The drawback of solvent extraction is that the conventional solvent chelating extraction method employs toxic organic solvents with residuals and back extraction treatment, which are complicated to handle. The drawback of ion exchange is that it is very costly, as the ion exchange resin is very expensive and reagents including acid, alkali and salt are required for resin regeneration. Thus, this method is rarely adopted for large-scale wastewater treatment projects. The weakness of membrane separation is that the membrane components are very hard to design and the membrane is very likely to get blocked by pollutants, thus requiring huge investment and high operating costs, which restricts application of the membrane separation method. (3) Biological treatment: it removes the heavy metal ions in wastewater by the flocculation, absorption, accumulation, and enrichment of microorganisms or plants, but it requires a certain period of time before beginning to show results. Therefore, it is unable to process large volume of wastewater instantly.26–28
According to the above analyses, the physical adsorption method is easy to use with little secondary pollution and recyclable adsorbents and has the advantage of processing heavy metal ions present in low concentrations. Recently, an important issue to be resolved for water resource protection is to find a conventional adsorbents such as activated carbon,29 metal oxides,30 clay minerals,31 which can effectively remove Cu and Ag metals.32,33 As the second major renewable resource material, chitosan is very suitable to be applied as a biomass adsorbent because of the amine and hydroxyl groups it possesses, and it can be administered with various application functions by grafting with different chemical reactions.34 In previously reported literature, common studies on chitosan mainly use α-chitosan generated from crabs and shrimps as the chemical modification material, but it has a low reaction grafting ratio.35,36 Therefore, this study adopted β-chitosan (BC) generated from the cartilage of cuttlefish as the chemical modification material, and benzaldehyde as the protecting group to convert the amine groups on location C2 of chitosan into a Schiff base. This was followed by grafting and crosslinking of epichlorohydrin and triethylenetetramine with chitosan and analyses of the structural changes with Fourier transform infrared spectroscopy (FTIR), UV-visible spectroscopy (UV-Vis), X-ray powder diffraction (XRD), elemental analyses (EA), nuclear magnetic resonance (NMR), thermogravimetric analysis (TGA), differential scanning calorimeter (DSC), Brunauer–Emmett–Teller (BET) surface area analysis and scanning electron microscope (SEM). Finally, this study compared the heavy metal ion adsorption capacities of chitosan before and after the graft modification in highly acidic environments.
2. Experimental
2.1 Materials
β-Chitosan (BC) with a deacetylation of 90% and molecular weight 500k D was obtained from Charming & Beauty, Taiwan. Benzaldehyde of reagent grade was obtained from TEDIA. Benzaldehyde of 99% reagent grade was from Alfa Aesar. Triethylenetetramine of 60% reagent grade was obtained from ACROS. Hydrochloric acid of 37% reagent grade was obtained from SCHARLAU. Silver nitrate (AgNO3, purity 99.9%) was obtained from Sigma-Aldrich Chemical Co. Copper(II) nitrate (Cu(NO3)2, purity 99.9%) was obtained from Sigma-Aldrich Chemical Co.
2.2 Preparation of crosslinked modified β-chitosan
2.2.1 Preparation of β-chitosan Schiff base (BCS). The first step in the modification reaction of BC in this study was to generate imine protecting groups by the reaction between benzaldehyde and the amine groups of BC. The experimental procedure was as follows. First, 1 g of chitosan powder was dissolved in 50 mL of 3% acetic acid. Then 50 mL of ethanol add to dissolve chitosan, followed by dropwise addition of 5 mL of benzaldehyde. After a 3 h reaction at a temperature of 60 °C, the products of the reaction were subjected to vacuum filtration after washing them repeatedly with ethanol and deionized water, and then purified with diethyl ether in the Soxhlet extractor for 12 h, followed by drying in an oven at a constant temperature of 90 °C for 12 h. Thus, in this manner the intermediate BCS was obtained.
2.2.2 Preparation of β-chitosan Schiff base epoxide (BCSE). The epichlorohydrin is a crosslinking agent with double reactivity functional groups. A ring-opening reaction can easily occur between the epoxide, hydroxyl, and amine groups under alkaline conditions, and the helium atoms in the epoxide molecules are also highly reactive functional groups. Epichlorohydrin is a modified form of chitosan. The experimental procedure was as follows. First, the reaction temperature was set as 65 °C. 100 mL of 0.4 mol L−1 lye, reactant mass ratio BCS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) epichlorohydrin of 1 g
epichlorohydrin of 1 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mL, and reaction time of 6 h were used to prepare the BCSE. The products were filtered and washed repeatedly with ethanol and deionized water to remove any unreacted epichlorohydrin. The finished products were dried in an oven at a constant temperature of 90 °C for 12 hour. Thus, the intermediate BCSE was obtained.
5 mL, and reaction time of 6 h were used to prepare the BCSE. The products were filtered and washed repeatedly with ethanol and deionized water to remove any unreacted epichlorohydrin. The finished products were dried in an oven at a constant temperature of 90 °C for 12 hour. Thus, the intermediate BCSE was obtained.
2.2.3 β-Chitosan Schiff base epoxide crosslinked triethylenetetramine (BCSECT). Following the last reaction step 2.2.2, the reaction temperature was set to 65 °C. 100 mL lye of 0.1 mol L−1, reactant mass ratio BCSE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) triethylenetetramine of 1 g
triethylenetetramine of 1 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mL, and reaction time of 6 h were employed to graft BCSE and triethylenetetramine. The products were filtered and washed repeatedly with ethanol and deionized water to remove any unreacted triethylenetetramine. The finished products were oven dried at a constant temperature of 90 °C for 12 h. Thus, the intermediate BCSECT was obtained.
3 mL, and reaction time of 6 h were employed to graft BCSE and triethylenetetramine. The products were filtered and washed repeatedly with ethanol and deionized water to remove any unreacted triethylenetetramine. The finished products were oven dried at a constant temperature of 90 °C for 12 h. Thus, the intermediate BCSECT was obtained.
2.2.4 β-Chitosan crosslinked triethylenetetramine (BCCT). Following the last reaction step 2.2.3, 1 N HCl solution was added to the synthesized BCSECT at room temperature and with stirring for 2 h. The product was filtered and washed successively with ethanol and deionized water. Then it was alkalified with 1 N NaOH solution for 2 h in case the amine groups on chitosan C2 got protonized and thereby weakened the heavy metal ion adsorption capacity of modified crosslinked chitosan. The products were filtered and washed with deionized water to neutralize them. The final products were dried in an oven at a constant temperature of 90 °C for 12 h. Thus, the intermediate BCCT was obtained.
2.3 Tests on the adsorption of heavy metal ions by crosslinked chitosan
A certain amount of metal salt was dissolved in 1 L of deionized water with different pH values (2, 3, 4, 5, and 6, respectively) and 1.5 mL of concentrated nitric acid was added in each liter to prepare 600 ppm sample solution of heavy metal ions. 0.1 g of pure BC and BCCT was added to 100 mL of the sample solution of heavy metal with different pH values and stirred for 1 h with a magnetic stirrer at room temperature. Then the adsorbent was removed and the heavy metal ion solution was collected and stored for AA analysis. The adsorbent was dried in an oven under 90 °C for 12 h for SEM analysis.
2.4 Characterization and instruments
Fourier transform infrared spectrometer was performed with a Spectrum One-Perkin Elmer. The synthesized BCCT and the intermediate products were dried and dewatered for 24 h in an oven at 100 °C. A trace amount of sample was ground in an agate mortar, then IR analytical grade KBr powder was added to it, and grinding was continued until they were mixed evenly. The mixture was pressed into a salt tablet and the FTIR spectrum was set for repeatedly scanning 32 times. Ultraviolet/visible light spectrophotometer was performed with a V-670-MODEL Spectrophotometer JASCO CORPORATION. The synthesized triethylenetetramine graft-modified chitosan and the intermediate products were dried and dewatered for 24 h in an oven at 100 °C. A certain amount of these was filled and fixed in a fixture and the measurement of ultra-violet absorption spectrum was set for repeatedly scanning 32 times. Thermogravimetric analyzer measurements were performed in a thermogravimetric analyzer Pyris 1 TGA-Perkin Elmer. 4–6 mg of synthesized triethylenetetramine graft-modified chitosan and the intermediate products were dewatered in a sample sink at 100 °C for 5 min, and then heated at rate of 10 °C min−1 in a scanning temperature range of 30 °C to 815 °C. Differential scanning calorimetry measurements were performed in a Pyris DSC-Perkin Elmer. 4–6 mg of synthesized triethylenetetramine graft-modified chitosan and the intermediate products were taken in a sample sink and heated at a heating rate of 10 °C min−1 in a scanning temperature range of 25 °C to 230 °C. Scanning electron microscope was performed in a HITACHI S-3000N. Samples of the synthesized triethylenetetramine graft-modified chitosan and the intermediate products were ground into fine powders in an agate mortar and then attached to the SEM holder for analyses. Elemental analyses were performed in a Thermo Flash 2000 Elemental Analysis. The synthesized triethylenetetramine graft-modified chitosan and the intermediate products were dewatered for 24 h in an oven at 100 °C. 3 mg to 5 mg of which material was folded into little cubes in a tin bag and kept in the automatic sample plate in order to analyze. X-ray powder diffraction was performed in a Rigaku (Japan) TTRAX III. The synthesized triethylenetetramine graft-modified chitosan and the intermediate products were ground into fine powder in an agate mortar, then scanned and analyzed continuously with an X-ray powder diffractometer. The analysis conditions were as follows: X-ray sources of Cu Kα (λ = 0.154 Å), output power of 30 kV and 20 mA, scanning speed of 2° min−1, and scanning range of 2θ = 5–55°. Solid state nuclear magnetic resonance spectrometer was performed in a Varian Inova AS500 MHz. The synthesized triethylenetetramine graft-modified chitosan and the intermediate products were ground into fine powders in an agate mortar, and then placed in an analyzer tube for 13C mapping analysis with Cross-polarization/Magic Angle Spinning. Atomic absorption spectrophotometer was performed in an Analyst 200-Perkin Elmer. A silver vacuum cathode lamp with a wavelength of 328.1 nm and a copper vacuum cathode lamp has a wave length of 324.8 nm, and a slit width of 0.7 nm were used.
3. Results and discussion
3.1 Synthesis and characterization of BCCT derivatives and their intermediate products
3.1.1 Analyses by Fourier infrared spectrometer. Scheme 1 shows the reaction formula of modified β-chitosan derivatives and their intermediate products. The synthetic reaction of the crosslinking modification of chitosan is to use BC as the main reactant and benzaldehyde as the protecting group to convert the amine groups on location C2 of chitosan into a Schiff base and then conduct grafting and crosslinking of epichlorohydrin and triethylenetetramine with chitosan to obtain crosslinked β-chitosan derivatives. Fig. 1 shows the FTIR test and the comparison of characteristic peaks of graft-modified β-chitosan derivatives and their intermediate products. The –C–O–C– absorption peak at 897 cm−1 and the glycosidic bond absorption peak at 1157 cm−1 remain unchanged during the entire modification reaction of β-chitosan, indicating that the pyranoid ring of β-chitosan and the –O– bond of the molecules always remain unchanged and are not degraded by the reaction. The difference between BC and BCS is that, after the Schiff base reaction, BCS exhibits the benzene ring mono-substitution absorption peaks at 692 and 757 cm−1 as well as the benzene ring C–H vibration absorption peaks at 859, 1453, and 1581 cm−1, with the absence of the –NH2 absorption peak at 1599 cm−1, indicating that the Schiff base reaction occurred between the C2–NH2 group of β-chitosan molecules and benzaldehyde.
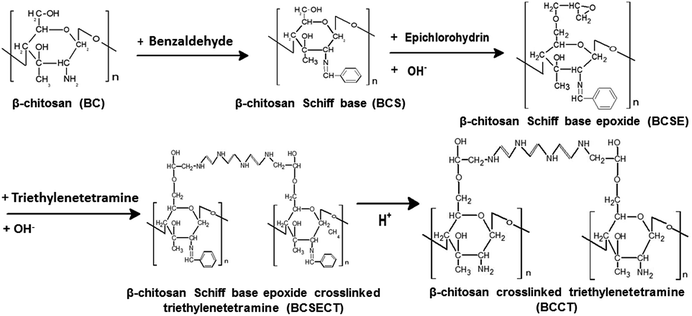 |
| | Scheme 1 Composition of β-chitosan crosslinked triethylenetetramine and its intermediate products. | |
 |
| | Fig. 1 FTIR analysis on the graft-modified chitosan and its intermediate products. | |
Regarding BCSE, the primary alcohol –OH absorption peak at 1025 cm−1, the secondary alcohol –OH absorption peak at 1068 cm−1, and the –OH and –NH2 absorption peaks at 3450 cm−1 weakened, indicating that the C6–OH of β-chitosan reacted with epichlorohydrin and an epoxide group was successfully grafted to β-chitosan molecules. After BCSECT formed a polyamine by ring opening, its absorption peaks of the primary alcohol –OH at 1025 cm−1, the secondary alcohol –OH at 1068 cm−1, and –OH and –NH2 at 3450 cm−1 became even weaker, indicating that a ring–opening reaction occurred for the grafted epoxide groups on β-chitosan molecules to successfully graft and crosslink the polyamine to β-chitosan molecules. After removing the protecting groups, disappearance of the benzene ring mono-substitution absorption peaks at 692 and 757 cm−1 as well as the benzene ring C–H vibration absorption peaks at 589, 1453 cm, and 1581 cm−1 occurred for BCCT. Along with appearance of the –NH2 absorption peaks at 1599 cm−1, indicating that the C2–NH2 group on β-chitosan molecules had successfully reduced benzaldehyde and removed the protecting groups.
3.1.2 Analyses by thermogravimetric analyzer. Fig. 2(a) shows the TGA curves of modified chitosan and its intermediate products. According to the TGA curves and the changes of the residual weight, the curve of each sample only revealed weight loss in a single stage, proving that chitosan at each modification stage was composed of a single product instead of mixtures. The cracking residual weight percentages of BC and BCCT were consistent with the results of the FTIR and UV-Vis analyses in each stage, exhibiting that the molecular structures were different in different modification steps and all the required molecular structures were introduced successfully in each step. The thermal cracking property indicates that the molecular structure of BCS contained benzene mono-substituted imine groups which, together with benzene rings, had double bonding and were very likely to cause bond fission and thus, resulted in a higher heat loss of weight percentage, confirming that benzaldehyde had been successfully introduced to the molecular structure. After the haloalkane was introduced, the molecular structure of BCSE generated the –O– bond of O-alkylate, and the –O– bond probably caused bond fission on heating and resulted in higher heat loss of weight percentage, proving that epichlorohydrin had been successfully introduced to the molecular structure. After the ring opening and crosslinking of the molecular structure of BCSECT with triethylenetetramine, the linear molecular chain of chitosan became a reticular structure, and the molecular structure required some extra energy for chain scission and thermal cracking, thus causing lower heat loss of weight percentage than BC, indicating the successful completion of the ring opening and crosslinking of triethylenetetramine. After the protecting groups were removed by acid, the heat loss of weight percentage of the molecular structure of BCCT reduced slightly in comparison with BC, indicating the successful removal of the protecting groups from the molecular structure and the completion of molecular modification.
 |
| | Fig. 2 (a) TGA and (b) DTG curves, and (c) DSC analytical curves of graft-modified chitosan and its intermediate products. | |
According to further analyses, Fig. 2(b) shows the DTG curves of graft-modified chitosan and its intermediate products. The changes of the curves of BC and BCCT prove that the molecular structures were different in different modification steps. All the required molecular structures were introduced successfully. The thermal cracking temperature improved by 8 °C after crosslinking and the thermal weight loss reduced slightly. It can be inferred from Table 1 that the molecular structure of BCS contained benzene mono-substituted imine groups which, together with benzene rings, had double bonding and were very likely to cause bond fission, and the required cracking temperature for the bond fission was 2 °C higher than that of BC, proving the successful introduction of benzaldehyde to the molecular structure. After haloalkane was introduced, the molecular structure of BCSE generated the –O– bond of O-alkylate, and the –O– bond can cause bond fission under heat with the required cracking temperature for the bond fission of 2 °C higher than that of b, proving the successful introduction of epichlorohydrin to the molecular structure. After the ring opening and crosslinking of the molecular structure of BCSECT with triethylenetetramine, the linear molecular chain of chitosan became a reticular structure, and the molecular structure required some extra energy for chain scission and thermal cracking with the required cracking temperature for the bond fission of 20 °C higher than that of BC, proving the successful completion of the ring opening and crosslinking of triethylenetetramine. After the protecting groups were removed by acid, the linear molecular chain of chitosan became a reticular structure, and the molecular structure required a some extra energy for chain scission and thermal cracking with the required temperature for chain scission and thermal cracking of 8 °C higher than that of the linear molecular chain of non-modified chitosan (BC), indicating the successful removal of the protecting groups from the molecular structure and the completion of molecular modification.
Table 1 Cracking residual weight of graft-modified chitosan and its intermediate products after TGA analysesa
| Sample |
BC |
BCS |
BCSE |
BCSECT |
BCCT |
| Cracking weight loss ratio = [(100% weight) − (residual weight%)] ÷ 100. |
| Weight loss ratio of cracking (%) under 600 °C |
62 |
64 |
66 |
59.55 |
61.21 |
| Cracking residual weight (%) under 600 °C |
38 |
36 |
34 |
40.45 |
38.79 |
| Derivative weight (% min−1) |
−0.4 |
−0.85 |
−0.55 |
−1.22 |
−0.35 |
| Thermal cracking temperature (°C) |
280 |
282 |
284 |
300 |
288 |
3.1.3 Analyses by differential scanning calorimeter. From the DSC analytical curves of graft-modified chitosan and its intermediate products shown in Fig. 2(c) it can be inferred that the non-modified chitosan molecules of BC were linear, and therefore, they have a high crystallinity; the grafting of BCS with phenyl groups disturbs the arrangement in the crystalline region, and thus, the Tm point of BCS was lower than that of the non-modified BC. The second type of side-chain molecules were introduced to the linear molecules of chitosan after BCSE was grafted with epichlorohydrin. The arrangement in the crystalline region was reduced again, and consequently, the Tm point of BCSE was lower than that of BCS. BCSECT had the poorest crystallinity, mainly because the linear molecular structure changed to a reticular structure after grafting and crosslinking triethylenetetramine. Furthermore, BCSECT also has a side-chain benzene ring structure, which also contributes to the reduction in crystallinity. The crystallinity of BCCT was also lower than before modification, mainly because its molecular structure is a reticular structure, and the side-chain phenyl groups were all removed by the acid. Thus, the crystallization Tm point of BCCT was the second highest. The melting point data is shown in Table 2. The change in the area under the peak indicates that crystallinity of chitosan decreased significantly with the progress in crosslinking modification. This was mainly because different molecular structures were introduced at different stages, and thus, a part of the crystalline region of chitosan was affected, resulting in the reduction in crystallinity.
Table 2 Tm of the DSC curves of graft-modified chitosan and its intermediate products
| Sample |
BC |
BCS |
BCSE |
BCSECT |
BCCT |
| Tm (°C) |
79.1 |
59.1 |
58.9 |
62.9 |
68.6 |
| Area (mJ) |
1046.8 |
421.9 |
403.2 |
429.1 |
839.5 |
| ΔH (J g−1) |
190.3 |
75.3 |
73.3 |
79.4 |
139.9 |
3.1.4 Elemental analyses. According to the elemental analyses in Table 3, the carbon content of BCS is higher than that of other samples, while the content of nitrogen, hydrogen and oxygen is lower than that of BC, because the phenyl groups introduced to chitosan molecules increased the carbon content, illustrating that the C2–NH2 groups were not involved in subsequent reactions after the Schiff base protecting groups were generated by chitosan and benzaldehyde. After the C2–NH2 groups of chitosan were under protection and the C6–OH in the molecules was epoxidized by epichlorohydrin, BCSE exhibited a small change in the carbon, nitrogen and hydrogen content in comparison with BCS, while the oxygen content increased more, in agreement with the successful introduction of epoxide groups to the molecular structure of chitosan. BCSECT's carbon and oxygen content reduced, while the nitrogen and hydrogen content improved slightly versus BCSE, proving the successful ring opening and crosslinking of triethylenetetramine and epoxide groups in this step. The carbon content of BCCT was 8.29 times lower than that of BCSECT, confirming the removal of amine protecting groups by hydrochloric acid on completion of the reaction. Additional, we further present the material characterizations of UV-Vis, XRD, and NMR, as shown in Fig. S1–S3 (see ESI for details†).
Table 3 Elemental analyses of graft-modified chitosan and intermediate products
| Sample |
Element (%) |
| C |
N |
H |
O |
Total |
C/N |
| BC |
41.72 |
7.77 |
8.06 |
40.63 |
98.18 |
5.37 |
| BCS |
55.40 |
5.20 |
6.29 |
31.20 |
98.09 |
10.65 |
| BCSE |
53.30 |
5.07 |
5.98 |
33.87 |
98.22 |
10.51 |
| BCSECT |
50.35 |
6.12 |
6.05 |
35.85 |
98.37 |
8.23 |
| BCCT |
42.06 |
7.78 |
8.08 |
40.40 |
98.32 |
5.41 |
3.2 Comparison of adsorption efficiencies of chitosan for heavy metal ions before and after modification
3.2.1 Analyses of adsorption capacity for copper and silver ions. Tables 4 and 5 show the comparison of adsorption efficiencies of chitosan for different heavy metal ions (copper and silver ions) before and after modification. The tables indicate that the adsorption capacity for copper and silver ions as well as the acid-resistant capability of graft-modified chitosan improved significantly than those of β-chitosan under different pH values, and the relation between the adsorption capacity Q and pH value is shown in Fig. 3 and S4.† The removal amount of Cu(II) and Ag(I) adsorbed per unit mass of adsorbent (Q) was calculated using the following eqn (1):37,38| |
 | (1) |
where Ci and C symbolize the initial concentration and equilibrium concentration in milligrams per liter, respectively. V is the volume of the solution in liters (L) and M is the mass of the adsorbent in grams.
Table 4 Adsorption efficiencies of β-chitosan and graft-modified chitosan for copper ions under different pH values
| Adsorbent |
BC |
BCCT |
| pH |
Initial concentration (ppm) |
Concentration after adsorption (ppm) |
Adsorption capacity Q (mg g−1) |
Initial concentration (ppm) |
Concentration after adsorption (ppm) |
Adsorption capacity Q (mg g−1) |
| 2 |
592 |
— |
— |
592 |
520 |
72.0 |
| 3 |
588 |
538 |
49.8 |
588 |
504 |
84.0 |
| 4 |
592 |
535 |
56.8 |
592 |
488 |
103.6 |
| 5 |
596 |
528 |
67.6 |
596 |
480 |
116.0 |
| 6 |
596 |
528 |
67.7 |
596 |
478 |
117.6 |
Table 5 Adsorption efficiencies of β-chitosan and graft-modified chitosan for silver ions under different pH values
| Adsorbent |
BC |
BCCT |
| pH |
Initial concentration (ppm) |
Concentration after adsorption (ppm) |
Adsorption capacity Q (mg g−1) |
Initial concentration (ppm) |
Concentration after adsorption (ppm) |
Adsorption capacity Q (mg g−1) |
| 2 |
579 |
— |
— |
579 |
498 |
80.8 |
| 3 |
577 |
520 |
57.6 |
577 |
448 |
128.9 |
| 4 |
580 |
471 |
108.8 |
580 |
436 |
144.0 |
| 5 |
578 |
462 |
116.0 |
578 |
432 |
146.4 |
| 6 |
576 |
460 |
116.8 |
576 |
425 |
151.2 |
 |
| | Fig. 3 Adsorption capacities of chitosan for (a) copper ions and (b) silver ions before and after modification. | |
Regarding the adsorption capacity under different pH values, chitosan was decomposed by acid when the pH value was lower and lost its adsorption capacity, and so, data measurement was impossible when the pH value was 2. The crosslinked chitosan was acid resistant as the linear molecular structure of chitosan became a reticular one after modification. Thus, the molecules were unlikely to be decomposed by acid and were well resistant to acid when the pH value was 2. Therefore, the crosslinked β-chitosan had an excellent acid-resistant property after modification. In addition, according to the comparison of the adsorption efficiencies of chitosan for copper and silver ions before and after modification, the adsorption efficiency for silver ions was higher and improved in acid environments with the pH value ranging from 2 to 6. Besides, the adsorption efficiency for silver ions improved from 67.76 mg g−1 to 117.60 mg g−1 when the pH value was 6. Furthermore, the adsorption capacity for silver ions was better than that for copper ions and improved from 116.80 mg g−1 to 151.20 mg g−1 when the pH value was 6 because the radii of silver ions are more suitable than that of copper ions for adsorption by chitosan.
3.2.2 Surface morphology observation of β-chitosan by scanning electron microscope. Fig. 4 shows the SEM surface morphology of β-chitosan before and after modification. Fig. 4(a) shows that the surface morphology of non-modified β-chitosan is smooth and dense; while that of each of the modified intermediates, shown in Fig. 4(b)–(d), is relatively rougher. The surface of the modified chitosan shown in Fig. 4(e) is also rough but with significant differences in appearance. The color of β-chitosan powder changed dramatically after adsorbing heavy metal ions before and after modification. In particular, the color turned deep blue after adsorbing copper ions. Fig. 5(a) and Video S1 (see ESI†) show the heavy metal ion adsorption of BCCT for copper ion before and after adsorption. Fig. 5(b) and (c) display the SEM images of the non-modified chitosan following adsorption of copper and silver ions, respectively. Fig. 5(d) and (e) exhibit the SEM images of graft-modified triethylenetetramine following copper and silver ion adsorption, respectively. Based on the surface microstructure morphology, the surface of BCCT, which was rougher than that of BC, demonstrated a higher adsorption capacity for ions, and chitosan before and after modification had a higher adsorption capacity for silver ions than for copper ions, causing the adsorption layer of silver ions on the surface to be thicker and denser than that of copper ions. Furthermore, the surface area and porosity of β-chitosan before and after modification with results summarized in Table S1.† The decrease in the surface area and porosity of the adsorbents appearing a more compact microstructure due to the crosslinking of triethylenetetramine with chitosan.
 |
| | Fig. 4 SEM surface morphology of graft-modified chitosan and its intermediate products: (a) shows the smooth surface morphology of non-modified β-chitosan (BC); (b–d) show the rough surface morphology of BCS, BCSE and BCSECT, respectively; and (e) shows the surface morphology of modified β-chitosan (BCCT) (inset: image of the corresponding graft-modified chitosan and its intermediate products). | |
 |
| | Fig. 5 (a) The heavy metal ion adsorption of BCCT for copper ion before and after adsorption. SEM of graft-modified chitosan and its intermediate products after adsorbing heavy metal: (b) shows the adsorption form of non-modified β-chitosan (BC) for copper ions; (c) shows the adsorption form of non-modified β-chitosan (BC) for silver ions; (d) shows the adsorption form of BCCT for copper ions; and (e) shows the adsorption form of BCCT for silver ions (inset: image of the corresponding adsorption forms of graft-modified chitosan and its intermediate products for heavy metals). | |
3.3 Adsorption mechanism of modified β-chitosan for ions
Scheme 2 shows the adsorption mechanism of modified β-chitosan for heavy metal ions. It can be inferred from the corresponding data analyses that β-chitosan adsorbs heavy metal ions mainly by capturing them by generating coordination bonds between heavy metal ions and the hydroxyl group and amine group lone pairs on chitosan. Alternately, the heavy metal ions are adsorbed by formation of coordination bonds with the amine group lone pairs on the crosslinked grafted polyamine. It can also be inferred from the previous experiment results that, for metals with different ionic valences, monovalent metal ions have more surface of adsorbents than divalent ions. Thus, this adsorption mechanism favors a higher adsorption capacity for monovalent silver ions than that for divalent copper ions. Such experimental results were also rational from the theoretical perspective. Therefore, this study successfully developed acid-resistant β-chitosan adsorbing materials for heavy metals.
 |
| | Scheme 2 Adsorption mechanism of crosslink-modified β-chitosan for monovalent (M+) and divalent (M2+) heavy metals. | |
4. Conclusion
Due to its causing severe pollution and environmental hazard, heavy metal wastewater can only be discharged after a series of complex treatments. Chitosan, which offers the advantages of biocompatibility, biodegradation and recyclability, can be adopted for heavy metal wastewater treatment. This study mainly conducted the crosslinking modification of β-chitosan and successfully analyzed its adsorption characteristics for different heavy metal ions in highly acidic environments. According to the results of the analyses on the modified β-chitosan and its intermediates, significant changes occurred in the characteristic absorption peaks of functional groups of FTIR, the absorption intensity of UV-Vis, the crystalline derivative peaks of XRD, the composition of EA, the thermal analytical curves as well as the surface microstructure morphology, proving that the obtained products were consistent with the designed products and the expected target has been achieved. The graft-modified β-chitosan had a many amine and imine groups in its molecules with a higher adsorption capacity for heavy metal ions and its resistance to the variation of pH values also improved. Before modification, the chitosan was not acid-resistant and dissolved completely in water solution with the pH value of 2, while the modified chitosan almost stayed the same under the same conditions. Therefore, this study successfully developed an acid-resistant heavy metal adsorbing material, which is expected to be applied for industrial wastewater treatment.
Acknowledgements
We acknowledge financial support from the Aim for the Top University Plan of the National Taiwan University of Science and Technology and the Ministry of Science and Technology (MOST 105-2221-E-011-155) of Taiwan.
References
- W. Cao, J. Zhang, Y. Wang, X. Zhang and M. Zhang, RSC Adv., 2015, 5, 32239–32244 RSC.
- P. Z. Ray and H. J. Shipley, RSC Adv., 2015, 5, 29885–29907 RSC.
- X. Yao, S. Deng, R. Wu, S. Hong, B. Wang, J. Huang, Y. Wang and G. Yu, RSC Adv., 2016, 6, 8797–8805 RSC.
- S. Peng, G. Jin, L. Li, K. Li, M. Srinivasan, S. Ramakrishna and J. Chen, Chem. Soc. Rev., 2016, 45, 1225–1241 RSC.
- H. N. M. Ekramul Mahmud, A. K. O. Huq and R. B. Yahya, RSC Adv., 2016, 6, 14778–14791 RSC.
- A. O. Olaniran, L. Hiralal and B. Pillay, J. Environ. Monit., 2011, 13, 2914–2920 RSC.
- S. A. El-Safty, M. A. Shenashen, M. Ismael, M. Khairy and M. R. Awual, Analyst, 2012, 137, 5278–5290 RSC.
- C. Song, W. Yang, N. Zhou, R. Qian, Y. Zhang, K. Lou, R. Wang and W. Wang, Chem. Commun., 2015, 51, 4443–4446 RSC.
- P. Singh, K. Chauhan, V. Priya and R. K. Singhal, RSC Adv., 2016, 6, 64946–64961 RSC.
- H. Chen, X. Zheng, Y. Chen and H. Mu, RSC Adv., 2013, 3, 9835–9842 RSC.
- Z. M. Lei, Q. D. An, Y. Fan, J. L. Lv, C. Gao, S. R. Zhai and Z. Y. Xiao, New J. Chem., 2016, 40, 1195–1204 RSC.
- S. Hosseini, H. N. M. Ekramul Mahmud, R. B. Yahya and I. Djordjevic, Mater. Lett., 2015, 149, 77–80 CrossRef CAS.
- P. V. Nidheesh, RSC Adv., 2015, 5, 40552–40577 RSC.
- J. Nonkumwong, S. Ananta and L. Srisombat, RSC Adv., 2016, 6, 47382–47393 RSC.
- J. Tang, B. Mu, W. Wang, M. Zheng and A. Wang, RSC Adv., 2016, 6, 36534–36543 RSC.
- H. Zhuang, S. Shan, C. Fang and X. Yuan, RSC Adv., 2016, 6, 39940–39946 RSC.
- J. C. Kwon, Z. D. Nejad and M. C. Jung, Catena, 2016 DOI:10.1016/j.catena.2016.01.005.
- N. Saha, M. Z. I. Mollah, M. F. Alam and M. S. Rahman, Food Control, 2016, 70, 110–118 CrossRef CAS.
- X. Liu, Q. Song, Y. Tang, W. Li, J. Xu, J. Wu, F. Wang and P. C. Brookes, Sci. Total Environ., 2013, 463, 530–540 CrossRef PubMed.
- R. L. Oulton, T. Kohn and D. M. Cwiertny, J. Environ. Monit., 2010, 12, 1956–1978 RSC.
- V. K. Gupta, I. Ali, T. A. Saleh, A. Nayak and S. Agarwal, RSC Adv., 2012, 2, 6380–6388 RSC.
- X. Yao, S. Deng, R. Wu, S. Hong, B. Wang, J. Huang, Y. Wang and G. Yu, RSC Adv., 2016, 6, 8797–8805 RSC.
- S. Karthikeyan, C. Anandan, J. Subramanian and G. Sekaran, RSC Adv., 2013, 3, 15044–15057 RSC.
- M. Theresa, M. Pendergast and E. M. V. Hoek, Energy Environ. Sci., 2011, 4, 1946–1971 Search PubMed.
- E. C. Su, B. S. Huang and M. Y. Wey, RSC Adv., 2016, 6, 71273–71281 RSC.
- W. W. Li, H. Q. Yu and Z. He, Energy Environ. Sci., 2014, 7, 911–924 CAS.
- X. Zhou, Y. Li and Y. Zhao, RSC Adv., 2014, 4, 15620–15629 RSC.
- W. Cao, RSC Adv., 2015, 5, 14042–14046 RSC.
- D. M. Zhou, Y. J. Wang, H. W. Wang, S. Q. Wang and J. M. Cheng, J. Hazard. Mater., 2010, 174, 34–39 CrossRef CAS PubMed.
- A. A. Ismail, A. A. El-Midany, I. A. Ibrahim and H. Matsunaga, Adsorption, 2008, 14, 21–29 CrossRef CAS.
- J. H. Potgieter, S. S. Potgieter-Vermaak and P. D. Kalibantonga, Miner. Eng., 2006, 19, 463–470 CrossRef CAS.
- J. C. Igwe, Terrestrial and Aquatic Environmental Toxicology, 2007, 1, 60–69 Search PubMed.
- S. Babel and T. A. Kurniawan, J. Hazard. Mater., 2003, 97, 219–243 CrossRef CAS PubMed.
- M. N. V. R. Kumar, React. Funct. Polym., 2000, 46, 1–27 CrossRef CAS.
- R. S. Juang and H. J. Shao, Adsorption, 2002, 8, 71–78 CrossRef CAS.
- H. Li, H. Li and Y. Liu, Iran. Polym. J., 2016, 25, 277–284 CrossRef CAS.
- S. Hasan, T. K. Ghosh, D. S. Viswanath and V. M. Boddu, J. Hazard. Mater., 2008, 152, 826–837 CrossRef CAS PubMed.
- J. Huang, Y. Zheng, L. Luo, Y. Feng, C. Zhang, X. Wang and X. Liu, J. Hazard. Mater., 2016, 306, 210–219 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: A movie depicting the show the heavy metal ion adsorption of BCCT for copper ion before and after adsorption. See DOI: 10.1039/c6ra21993d |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) epichlorohydrin of 1 g
epichlorohydrin of 1 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mL, and reaction time of 6 h were used to prepare the BCSE. The products were filtered and washed repeatedly with ethanol and deionized water to remove any unreacted epichlorohydrin. The finished products were dried in an oven at a constant temperature of 90 °C for 12 hour. Thus, the intermediate BCSE was obtained.
5 mL, and reaction time of 6 h were used to prepare the BCSE. The products were filtered and washed repeatedly with ethanol and deionized water to remove any unreacted epichlorohydrin. The finished products were dried in an oven at a constant temperature of 90 °C for 12 hour. Thus, the intermediate BCSE was obtained.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) triethylenetetramine of 1 g
triethylenetetramine of 1 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mL, and reaction time of 6 h were employed to graft BCSE and triethylenetetramine. The products were filtered and washed repeatedly with ethanol and deionized water to remove any unreacted triethylenetetramine. The finished products were oven dried at a constant temperature of 90 °C for 12 h. Thus, the intermediate BCSECT was obtained.
3 mL, and reaction time of 6 h were employed to graft BCSE and triethylenetetramine. The products were filtered and washed repeatedly with ethanol and deionized water to remove any unreacted triethylenetetramine. The finished products were oven dried at a constant temperature of 90 °C for 12 h. Thus, the intermediate BCSECT was obtained.







