Zn2−aGeO4:aRE and Zn2Ge1−aO4:aRE (RE = Ce3+, Eu3+, Tb3+, Dy3+): 4f–4f and 5d–4f transition luminescence of rare earth ions under different substitution
Qiongyu Bai,
Zhijun Wang*,
Panlai Li*,
Shuchao Xu,
Ting Li and
Zhiping Yang
College of Physics Science & Technology, Hebei Key Lab of Optic-Electronic Information and Materials, Hebei University, Baoding 071002, China. E-mail: wangzj1998@126.com; li_panlai@126.com
First published on 10th October 2016
Abstract
Generally, luminescent properties of rare earth ions doped host can be tuned by controlling the host composition, that is, when substituted for different cations of host, the rare earths ions can present different characteristics. In this research, Zn2−xGeO4:xCe3+ and Zn2Ge1−xO4:xCe3+ can show two different emission bands due to the 5d–4f transitions of Ce3+ ions. Moreover, the CIE chromaticity coordinates and the luminescence photographs of Zn2−xGeO4:xCe3+ and Zn2Ge1−xO4:xCe3+ exhibit different characteristics. The emission bands appear blueshift and redshift due to the combination of crystal field splitting and nephelauxetic effect. However, for Zn2−aGeO4:aR and Zn2Ge1−aO4:aR (R = Eu3+, Tb3+, Dy3+), the 4f–4f characteristic transitions of Tb3+, Eu3+ and Dy3+ are observed, and the emission and excitation peaks are not influenced by the crystal field.
1 Introduction
Recently, tuning luminescence appearance has been widely investigated, that is, to tune the spectral position and shape and improve the thermal stability and the luminescence efficiency of phosphors.1,2 Many strategies can be used, such as chemical unit cosubstitution, neighboring-cation substitution, cation-size-mismatch, nanosegregation and neighbor-cation control, mixing of nanophases, site-preferential occupancy, and so on.3–9 Herein, most of the strategies are the control of substituted cations, they can exhibit different luminescence when the doping ions substitute for the cations of different position. For example, the superposition of emission spectra that cover the entire visible spectrum can be obtained due to that Ce3+ ions substitute for two different sites of Ca2+ sites (eight- and seven-coordination) in Ca4F2Si2O7 phosphor.10 (Ca0.993−x−yMgxSry)9Y(PO4)7:0.007Eu2+ can show a continuous blueshift emission with increasing Mg2+ concentration and a redshift with increasing Sr2+ level.11 Therefore, the effect of substitution on luminescence is worth being explored.There are some reports focused on the material Zn2GeO4 due to its excellent properties, such as the simplicity of the crystal structure, high stability, decomposition of water, negative thermal expansion and electroluminescence.12–16 Zn2GeO4 with a wide band gap (4.4 eV) has potential application in photocatalysts, optoelectronic devices and displays.17–24 Zn2GeO4:Eu3+ nanocrystals show a white light emission, which displays a potential application for white light-emitting diodes (white LEDs).25 Cr3+, Yb3+, Er3+ doped zinc gallogermanate nanoparticles exhibit an excellent superlong near-infrared persistent luminescence.26 With respect to the above, we select Zn2GeO4 as host, and attempt to investigate the effect of rare earths ions on luminescence properties. We select Eu3+, Tb3+, Dy3+ based on 4f–4f transition and crystal field sensitive Ce3+ (5d–4f transition) as doping ions, and Zn2−aGeO4:aRE3+ and Zn2Ge1−xO4:aRE3+ (RE = Ce3+, Eu3+, Tb3+, Dy3+) were synthesized using a high temperature solid-state method. There is a blueshift and redshift can be obtained when Ce3+ doped in Zn2GeO4, due to that positions of the 5d levels are greatly influenced by the crystal field. We find that the emission and excitation peaks are not influenced, however, the relative emission and excitation intensities of phosphors can be tuned when Eu3+, Tb3+, Dy3+ substitute for Zn2+ and Ge4+ in Zn2GeO4.
2 Experimental
2.1 Sample preparation
A series of phosphors with composition Zn2−aGeO4:aRE3+ and Zn2Ge1−aO4:aRE3+ (RE = Ce3+, Eu3+, Tb3+, Dy3+) were synthesized by a high-temperature solid-state method. The starting materials, including reagent grade ZnO, GeO2 (99.99%), CeO2 (99.99%), Eu2O3 (99.99%), Tb4O7 (99.99%), Dy2O3 (99.99%), were weighed in stoichiometric proportion, mixed homogeneously and ground by an agate mortar. The resultant mixtures are heated up to 850 °C held for 2 h in air, and then cooled down to room temperature naturally with the furnace. After intermediately ground to improve the homogeneity, the mixtures were sintered at 1350 °C for 2.5 h in air to get the final phosphors.2.2 Characterization
The phase formation of samples were characterized by X-ray powder diffraction (XRD) performed on a Bruker D8 X-ray diffractometer with Ni-filtered Cu Kα radiation (λ = 0.15405 nm), operating at 40 mA, 40 kV. The XRD data for Rietveld structure analysis were collected over the 2θ ranges from 10° to 80° in a step-mode with step length were 0.02°. To analyze the chemical composition of phosphors, electron-dispersive X-ray (EDX) data were collected by a Nova NanoSEM 650 with an accelerating voltage of 10 kV. Furthermore, the scanning electron micrograph (SEM) was used to collect images for investigating particle morphology. Room-temperature photoluminescence spectra of samples were recorded with a Hitachi F-4600 fluorescence spectrophotometer using a 450 W Xe lamp as the excitation source, with a scanning wavelength from 200 to 700 nm, scanning at 240 nm min−1. The diffuse reflection (DR) spectra were measured by a Hitachi U4100 UV-VIS-NIR spectroscopy using BaSO4 as a reference, with a scanning wavelength from 200 to 700 nm. Luminescence decay curves of samples were collected by a Horiba FL-4600 fluorescence spectrophotometer using a Xe lamp as the excitation source. Luminescence decay curves of Ce3+ were measured using a 265 nm nano-LED as the excitation source. The Commission International de I'Eclairage (CIE) coordinates for all samples were recorded with a PMS-80 UV-VIS-NEAR IR spectra analysis system.3 Results and discussion
3.1 Phase formation and structure
The XRD patterns of Zn2−aGeO4:aRE and Zn2Ge1−aO4:aRE (RE = Ce3+, Eu3+, Tb3+, Dy3+) are measured, and similar diffraction patterns are observed for each sample. As a representative, we only select the results of Zn1.99GeO4:0.01RE and Zn2Ge0.99O4:0.01RE. The XRD patterns of Zn1.99GeO4:0.01Ce3+ and Zn2Ge0.99O4:0.01Ce3+ are used to perform the Rietveld refinement with General Structure Analysis System (GASA) software,27 as shown in Fig. 1a and b, respectively. All of samples are verified to comprise a single phase and they are consistent with the standard file of Zn2GeO4 (JCPDS no. 11-0687). Zn2GeO4 shares a hexagonal structure with space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , a = b = 14.231 Å, c = 9.53 Å, α = β = 90°, γ = 120° and V = 1671.4 Å3, and the structure of Zn2GeO4 viewed from [001] is shown in the inset of Fig. 1a. There are three crystallographic positions of cations in the unit cell: two independent 4-fold coordinated Zn2+ sites and a 4-fold coordinated Ge4+ site in the crystal structure of Zn2GeO4, as shown in the inset of Fig. 1b. Fig. 2 illustrates that the representative XRD patterns of rare earth ions R (R = Eu3+, Tb3+, Dy3+) substitute for Zn2+ and Ge4+ in Zn2GeO4, respectively, as well as the standard pattern of JCPDS no. 11-0687. It is apparent that all the diffraction peaks are well assigned to the pure phase of Zn2GeO4 without any impurity.
, a = b = 14.231 Å, c = 9.53 Å, α = β = 90°, γ = 120° and V = 1671.4 Å3, and the structure of Zn2GeO4 viewed from [001] is shown in the inset of Fig. 1a. There are three crystallographic positions of cations in the unit cell: two independent 4-fold coordinated Zn2+ sites and a 4-fold coordinated Ge4+ site in the crystal structure of Zn2GeO4, as shown in the inset of Fig. 1b. Fig. 2 illustrates that the representative XRD patterns of rare earth ions R (R = Eu3+, Tb3+, Dy3+) substitute for Zn2+ and Ge4+ in Zn2GeO4, respectively, as well as the standard pattern of JCPDS no. 11-0687. It is apparent that all the diffraction peaks are well assigned to the pure phase of Zn2GeO4 without any impurity.
 | ||
| Fig. 1 X-ray Rietveld refinement for Zn1.99GeO4:0.01Ce3+ (a) and Zn2Ge0.99O4:0.01Ce3+ (b), the inset shows the crystal structure of Zn2GeO4. | ||
Table 1 shows the cell parameters and volumes of Zn1.99GeO4:0.01RE and Zn2Ge0.99O4:0.01RE (RE = Ce3+, Eu3+, Tb3+, Dy3+). It is seen that the cell parameters and volumes of Zn1.99GeO4:0.01RE and Zn2Ge0.99O4:0.01RE are larger than that of Zn2GeO4, due to that the radii of RE are larger than that of Zn2+ and Ge4+. For the Zn2+ and Ge4+ ions, the ionic radii are 0.60 Å (Zn2+, CN = 4), and 0.58 Å (Ge4+, CN = 4), respectively. Therefore, the cell parameters and volumes of Zn2Ge0.99O4:0.01RE are larger than that of Zn1.99GeO4:0.01RE because the radius of Ge4+ is smaller than that of Zn2+. SEM images of Zn1.99GeO4:0.01RE and Zn2Ge0.99O4:0.01RE are measured and the similar shapes are observed for each sample. As a representative, Fig. 3 shows the SEM images of Zn1.99GeO4:0.01Ce3+ and Zn2Ge0.99O4:0.01Ce3+. It is clearly seen that these samples have ball shapes with micrometer size, and the EDX spectra confirm the presence of Zn, Ge, Ce and O in Zn1.99GeO4:0.01Ce3+ and Zn2Ge0.99O4:0.01Ce3+.
| Sample | RE | Cell parameters | Volume | |
|---|---|---|---|---|
| a/b (Å) | c (Å) | V (Å3) | ||
| Zn1.99GeO4:0.01RE | Ce3+ | 14.238 | 9.528 | 1672.83 |
| Eu3+ | 14.238 | 9.524 | 1671.06 | |
| Tb3+ | 14.238 | 9.526 | 1672.40 | |
| Dy3+ | 14.238 | 9.525 | 1672.38 | |
| Zn2Ge0.99O4:0.01RE | Ce3+ | 14.275 | 9.550 | 1685.50 |
| Eu3+ | 14.259 | 9.541 | 1680.10 | |
| Tb3+ | 14.260 | 9.545 | 1680.83 | |
| Dy3+ | 14.247 | 9.535 | 1676.05 | |
3.2 Luminescent property
In order to further verify the influence of host compositions on luminescence and crystal field, we select Ce3+ as the doping ion, due to that the positions of the 4fn−15d levels are greatly influenced by the crystal field interaction. According to our previous reports,28 Zn2GeO4 exhibits a broad emission band ranges from 350 to 650 nm due to the existence of O vacancy , single ionized Zn interstitial
, single ionized Zn interstitial 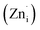 , Ge vacancy (VGe) and ionized Zn
, Ge vacancy (VGe) and ionized Zn 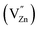 , and the excitation band locates at 270 nm. The diffuse reflection (DR) spectra of Zn1.92GeO4:0.08Ce3+ and Zn2Ge0.92O4:0.08Ce3+ are shown in Fig. 4a and b, respectively, it can be seen that the absorption bands extending from 200 to 350 nm are attributed to the native defects of host and 4f–5d transitions of Ce3+ ion. The excitation spectra (λem = 510/515 nm) show a absorption band extending from 200 to 300 nm with the maxima at 270 nm, which are attributed to the electronic transition from the 4f ground state to the 5d excited state of the Ce3+ ion.29,30 Besides that, the excitation spectrum of Zn1.92GeO4:0.08Ce3+ exhibits another band, peaking at 310 nm monitored at 510 nm, which is due to that Ce3+ ions occupy two independent 4-fold coordinated Zn2+ sites, as shown in the inset of Fig. 4a. Meanwhile, DR spectra ranging from 300 to 350 nm show different trends. Under 270 nm excitation, an emission band of Zn1.92GeO4:0.08Ce3+ was fitted by a sum of three Gaussian peaks in which two are attributed to the Ce3+ band centered at 459 (21
, and the excitation band locates at 270 nm. The diffuse reflection (DR) spectra of Zn1.92GeO4:0.08Ce3+ and Zn2Ge0.92O4:0.08Ce3+ are shown in Fig. 4a and b, respectively, it can be seen that the absorption bands extending from 200 to 350 nm are attributed to the native defects of host and 4f–5d transitions of Ce3+ ion. The excitation spectra (λem = 510/515 nm) show a absorption band extending from 200 to 300 nm with the maxima at 270 nm, which are attributed to the electronic transition from the 4f ground state to the 5d excited state of the Ce3+ ion.29,30 Besides that, the excitation spectrum of Zn1.92GeO4:0.08Ce3+ exhibits another band, peaking at 310 nm monitored at 510 nm, which is due to that Ce3+ ions occupy two independent 4-fold coordinated Zn2+ sites, as shown in the inset of Fig. 4a. Meanwhile, DR spectra ranging from 300 to 350 nm show different trends. Under 270 nm excitation, an emission band of Zn1.92GeO4:0.08Ce3+ was fitted by a sum of three Gaussian peaks in which two are attributed to the Ce3+ band centered at 459 (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 786 cm−1) nm and 510 (19
786 cm−1) nm and 510 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 608 cm−1) nm with an energy difference of 2178 cm−1, and another Gaussian peak is assigned to the Zn2GeO4 host emission band centered at 419 nm. The emission spectra of Zn2Ge0.92O4:0.08Ce3+ was deconvoluted using Gaussian profiles into two Gaussian peaks centered at 463 (21
608 cm−1) nm with an energy difference of 2178 cm−1, and another Gaussian peak is assigned to the Zn2GeO4 host emission band centered at 419 nm. The emission spectra of Zn2Ge0.92O4:0.08Ce3+ was deconvoluted using Gaussian profiles into two Gaussian peaks centered at 463 (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 598 cm−1) nm and 515 (19
598 cm−1) nm and 515 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 418 cm−1) nm with an energy difference of 2180 cm−1 and another Gaussian peak at 420 nm, which is attributed to the Zn2GeO4 host. The energy differences of Zn1.92GeO4:0.08Ce3+ and Zn2Ge0.92O4:0.08Ce3+ are found to be in good agreement with the theoretical energy value (2000 cm−1), due to the ground state splitting between the 2F7/2 and 2F5/2 levels of Ce3+ ions.31
418 cm−1) nm with an energy difference of 2180 cm−1 and another Gaussian peak at 420 nm, which is attributed to the Zn2GeO4 host. The energy differences of Zn1.92GeO4:0.08Ce3+ and Zn2Ge0.92O4:0.08Ce3+ are found to be in good agreement with the theoretical energy value (2000 cm−1), due to the ground state splitting between the 2F7/2 and 2F5/2 levels of Ce3+ ions.31
 | ||
| Fig. 4 Diffuse reflection (DR), emission and excitation spectra of Zn1.92GeO4:0.08Ce3+ (a) and Zn2Ge0.92O4:0.08Ce3+ (b), the inset shows the diagram of substitution. | ||
The emission intensities of the three Gaussian peaks as a function of doping concentration (x) in the Zn2−xGeO4:xCe3+ and Zn2Ge1−xO4:xCe3+ are illustrated in Fig. 5a–f, respectively. The emission intensities first increase and then decrease with the doping level, and the optimal values of 0.01 and 0.03 are obtained for Zn2−xGeO4:xCe3+ and Zn2Ge1−xO4:xCe3+, respectively. The emission intensities decrease due to the concentration quenching effect. The concentration quenching characteristics of Zn2−xGeO4:xCe3+ and Zn2Ge1−xO4:xCe3+ can be further understood by evaluating the critical distance (Rc) of Ce3+ ions. The Rc value can be estimated by following formula:32
 | (1) |
Fig. 6a–f show the normalized spectra of Zn2−xGeO4:xCe3+ at Peak 1 = 419 nm, Peak 2 = 459 nm, Peak 3 = 510 nm and Zn2Ge1−xO4:xCe3+ at Peak 4 = 420 nm, Peak 5 = 463 nm, Peak 6 = 515 nm, respectively. For the host emission bands (Peak 1 and Peak 4), they show blueshift at first and then appear redshift while the concentration of Ce3+ increases, which illustrates that the native defects have influence on the doping ions due to mismatched valence. For Zn2−xGeO4:xCe3+, the emission bands of Peak 2 and Peak 3 shift to the lower wavelength for x ≤ 0.02. The blueshift could be explained by a higher 4f6d1 band position due to the nephelauxetic effect with doping ions increasing.34,35 The electronegativity of Zn, Ge and Ce are 1.65, 2.01 and 1.12, respectively. The difference of electronegativity between cation and anion increases due to that the electronegativity of Ce is lower than that of Zn and Ge. Hence, the covalency of Zn2−xGeO4:xCe3+ decreases with increase Ce3+ concentration, which causes a blueshift of Ce3+ emission due to the nephelauxetic effect. However, the Ce3+ emission bands appear a redshift for x > 0.02 due to the crystal field spitting effect. Generally, the crystal field splitting (Dq) trends with bond length can be determined by the following equation:36–39
 | (2) |
 | ||
| Fig. 6 Normalized spectra of Zn2−xGeO4:xCe3+ at Peak 1 = 419 nm (a), Peak 2 = 459 (b) nm, Peak 3 = 510 nm (c) and Zn2Ge1−xO4:xCe3+ at Peak 4 = 420 nm (d), Peak 5 = 463 (e) nm, Peak 6 = 515 nm (f). | ||
For Zn2Ge1−xO4:xCe3+, the emission bands of Peak 5 and 6 show a blueshift for x ≤ 0.01, and then appear a redshift with the increment of doping concentration. However, the emission band of Peak 6 again shows a blueshift for x ≥ 0.05. The reason is that there are two effects influence the shift, that is, blueshift and redshift are due to that nephelauxetic effect and crystal field spitting effect are the dominant type, respectively. As compared to Zn2−xGeO4:xCe3+, the different shift is caused by different crystal field splitting. Ce3+ ions doped in Zn2−xGeO4:xCe3+ and Zn2Ge1−xO4:xCe3+ by substituting Zn2+ and Ge4+ ions, because the radius of Ge4+ (0.58 Å) is smaller than that of Zn2+ (0.60 Å), which results in a smaller crystal field splitting in Zn2Ge1−xO4:xCe3+.
Fig. 8a and b show the decay curves of Zn2−xGeO4:xCe3+ and Zn2Ge1−xO4:xCe3+ monitored at 419 and 420 nm, respectively, and the lifetime (τ) of host luminescence can be expressed as40
 | (3) |
| I(t) = I0 + A1e−t/τ1 + A2e−t/τ2 | (4) |
| τav = (A1τ12 + A2τ22)/(A1τ1 + A2τ2) | (5) |
 | ||
| Fig. 8 Decay curves of Zn2−xGeO4:xCe3+ monitored at 419 nm (a) and Zn2Ge1−xO4:xCe3+ monitored at 420 nm (b). | ||
 | ||
| Fig. 9 Decay curves of Zn2−xGeO4:xCe3+ monitored at 510 nm (a) and Zn2Ge1−xO4:xCe3+ monitored at 515 nm (b). | ||
It can be seen that the lifetimes increase first, and then decrease with the increasing Ce3+ doping concentration due to the concentration quenching, which is matched well with the emission spectra characteristics. The lifetimes of Zn2Ge1−xO4:xCe3+ are larger than that of Zn2−xGeO4:xCe3+, because Zn2Ge1−xO4:xCe3+ are in a relatively weak crystal field. The reason is that Ce3+ ions substitute for Zn2+ and Ge4+ ions in Zn2−xGeO4:xCe3+ and Zn2Ge1−xO4:xCe3+, and the radius of Ge4+ is smaller than that of Zn2+.
As shown in Fig. 10, the CIE chromaticity coordinates and corresponding photographs of the Zn2−xGeO4:xCe3+ and Zn2Ge1−xO4:xCe3+ (x = 0.01, 0.03 and 0.05) under 254 nm UV lamps. The luminescence colors of Zn2−xGeO4:xCe3+ and Zn2Ge1−xO4:xCe3+ are blue and blue green, respectively. The photographs matched with their color tones, which are consistent with the emission spectra.
 | ||
| Fig. 10 CIE chromaticity diagram of Zn2−xGeO4:xCe3+ and Zn2Ge1−xO4:xCe3+ (x = 0.01, 0.03 and 0.05) and photographs under 254 nm UV lamps. | ||
The luminescence of different doping level for R (R = Eu3+, Tb3+, Dy3+) doped Zn2−aGeO4 and Zn2Ge1−aO4 are measured, and similar luminescence characteristics are observed for each sample. As a representative, we only select the results of a = 0.08. Fig. 11 exhibits the diffuse reflection (DR), excitation and emission spectra of Zn1.92GeO4:0.08Eu3+, Zn2Ge0.92O4:0.08Eu3+, Zn1.92GeO4:0.08Tb3+, Zn2Ge0.92O4:0.08Tb3+, Zn1.92GeO4:0.08Dy3+ and Zn2Ge0.92O4:0.08Dy3+. It can be seen from Fig. 11a and b that all of the excitation spectra are consistent with the DR spectra. The excitation spectra of Zn1.92GeO4:0.08Eu3+, Zn2Ge0.92O4:0.08Eu3+ (λem = 620 nm) show a broad absorption band peaking at 270 nm, which is attributed to charge-transfer band (CTB) between Eu3+ and the surrounding oxygen ions,42–44 and other sharp bands are due to the 7F0 → 5L6, 5D2, 5D1 transition of Eu3+. Comparing to the excitation spectra of Zn1.92GeO4:0.08Eu3+ and Zn2Ge0.92O4:0.08Eu3+, it can be seen that the ratio of excitation bands intensities are changed, which is caused by the different crystal field due to different host compositions. In addition, Eu3+ doped Zn1.92GeO4 and Zn2Ge0.92O4 show the similar emission peaks, including a broad Zn2GeO4 host emission band peaking at 430 nm and several bands with peaks at 584, 594, 615 and 625 nm due to the 5D0 → 7FJ (J = 0, 1, 2, 3) transition of Eu3+. For Zn1.92GeO4:0.08Tb3+ and Zn2Ge0.92O4:0.08Tb3+, the excitation bands peaking at 257 nm are attributed to the spin-allowed transition from the 4f to the 5d state of Tb3+, and 270 nm band is ascribed to Zn2GeO4 host due to the energy transfer from Zn2GeO4 to Tb3+,28 as shown in Fig. 11c and d. Under excitation of 270 nm, phosphors show a host emission band peaking at 430 nm, and four emission bands centered at 494, 554, 590 and 629 nm due to the 5D4 → 7F6, 7F5, 7F4, 7F3 transitions of Tb3+, respectively. Zn1.92GeO4:0.08Tb3+ and Zn2Ge0.92O4:0.08Tb3+ exhibit similar emission and excitation bands due to the energy transfer. Fig. 11e and f show the excitation spectra of Zn1.92GeO4:0.08Dy3+ and Zn2Ge0.92O4:0.08Dy3+, and the excitation spectra consist of a CTB of Dy3+ centered at 270 nm and two bands centered at 315 and 385 nm, corresponding to the transition from 6H15/2 to 6P3/2 and 4F7/2, respectively. The emission band centered at 430 nm is attributed to the Zn2GeO4 host, and other bands peaking at 530 and 586 nm are due to 4F9/2 → 6H13/2 transition of Dy3+. The 4F9/2 → 6H13/2 transition is corresponding to the forced electric dipole transition (J = 2), therefore it is strongly influenced by the chemical environment surrounding of Dy3+.45 Hence, the 530 nm emission band is caused by the Stark energy splitting. There is an obvious difference observed by comparing Dy3+ doped Zn1.92GeO4 and Zn2Ge0.92O4 that the relative intensity of emission band peaking at 530 m in Zn1.92GeO4:0.08Dy3+ is greater than that of in Zn2Ge0.92O4:0.08Dy3+, which is caused by the different crystal field environment due to different host compositions. To summarize, there is no influence on the position of emission and excitation bands, due to that the RE ions based on 4f–4f transitions (Tb3+, Eu3+, Dy3+) are less affected by the crystal field environment.
Fig. 12a–f show the decay curves of Zn1.92GeO4:0.08Eu3+ (λem = 620 nm), Zn2Ge0.92O4:0.08Eu3+ (λem = 620 nm), Zn1.92GeO4:0.08Tb3+ (λem = 554 nm), Zn2Ge0.92O4:0.08Tb3+ (λem = 554 nm), Zn1.92GeO4:0.08Dy3+ (λem = 586 nm) and Zn2Ge0.92O4:0.08Dy3+ (λem = 586 nm) when specimens were excited at 270 nm, respectively, the lifetimes can be calculated using formula (3). The corresponding lifetimes are calculated to be 3.51 ms, 3.37 ms, 163.82 μs, 157.27 μs, 4.23 ms and 3.99 ms for Zn1.92GeO4:0.08Eu3+, Zn2Ge0.92O4:0.08Eu3+, Zn1.92GeO4:0.08Tb3+, Zn2Ge0.92O4:0.08Tb3+, Zn1.92GeO4:0.08Dy3+ and Zn2Ge0.92O4:0.08Dy3+, respectively. By comparison, we found that Eu3+, Tb3+, Dy3+ doped Zn1.92GeO4 and Zn2Ge0.92O4 have similar lifetimes, which are not influenced by the crystal field environments.
 | ||
| Fig. 12 Decay curves of Zn1.92GeO4:0.08Eu3+ (a), Zn2Ge0.92O4:0.08Eu3+ (b), Zn1.92GeO4:0.08Tb3+ (c), Zn2Ge0.92O4:0.08Tb3+ (d), Zn1.92GeO4:0.08Dy3+ (e) and Zn2Ge0.92O4:0.08Dy3+ (f). | ||
Fig. 13 shows the CIE chromaticity coordinates and corresponding photographs of Zn1.92GeO4:0.08Eu3+, Zn2Ge0.92O4:0.08Eu3+, Zn1.92GeO4:0.08Dy3+, Zn2Ge0.92O4:0.08Dy3+, Zn1.92GeO4:0.08Tb3+ and Zn2Ge0.92O4:0.08Tb3+ (point 1–6) under 254 nm UV lamps. It can be seen that the luminescence colors of Zn1.92GeO4:0.08Eu3+, Zn2Ge0.92O4:0.08Eu3+ are blue, which is due to that the intensity of host emission is larger than that of Eu3+ ions. The luminescence colors of Zn1.92GeO4:0.08Dy3+, Zn2Ge0.92O4:0.08Dy3+ are green and blue green, respectively. The reason of different colors is that the 530 nm intensity of Dy3+ for Zn1.92GeO4:0.08Dy3+ is stronger than that for Zn2Ge0.92O4:0.08Dy3+. Zn1.92GeO4:0.08Tb3+ and Zn2Ge0.92O4:0.08Tb3+ show similar green colors, due to the energy transfer from Zn2GeO4 host to Tb3+. In addition, their corresponding photographs are consistent with the color tones.
4 Conclusions
Zn2−aGeO4:aRE3+ and Zn2Ge1−aO4:aRE3+ (RE = Ce3+, Eu3+, Tb3+, Dy3+) with different compositions were synthesized by a high-temperature solid-state method. The increase in cell parameters and volumes with the substitution of RE for Zn2+ and Ge4+ confirms the formation of phosphors. For the Ce3+ ions based on 5d–4f transition, Zn1.92GeO4:0.08Ce3+ and Zn2Ge0.92O4:0.08Ce3+ show two emission bands centered at 459, 510 nm and 463, 515 nm, respectively. The CIE chromaticity coordinates and the luminescence photographs of Zn2−xGeO4:xCe3+ and Zn2Ge1−xO4:xCe3+ show different characteristics. The emission bands show blueshift and redshift with the increment of Ce3+ ions concentration, and the blueshift and redshift are caused by the nephelauxetic effect and crystal field splitting, respectively. For Zn2−aGeO4:aR and Zn2Ge1−aO4:aR (R = Tb3+, Eu3+, Dy3+), the positions of emission and excitation bands are not influenced by the crystal field, however the relative intensities of each emission and excitation bands are changed. The reason is that the rare earth ions based on 4f–4f transitions are less influenced by the crystal field environment. The proposed luminescence tuning can be realized by controlling host composition, and it is potential to extend to other phosphors.Acknowledgements
The work is supported by the National Natural Science Foundation of China (No. 50902042), the Funds for Distinguished Young Scientists of Hebei Province, China (No. A2015201129), the Natural Science Foundation of Hebei Province, China (No. A2014201035, E2014201037), the Education Office Research Foundation of Hebei Province, China (No. ZD2014036, QN2014085), China Postdoctoral Science Foundation funded project (No. 2015M581311), and the Midwest Universities Comprehensive Strength Promotion Project.References
- F. Kang, H. Zhang, L. Wondraczek, X. Yang, Y. Zhang, D. Y. Lei and M. Peng, Chem. Mater., 2016, 28, 2692–2703 CrossRef CAS.
- F. Kang, X. Yang, M. Peng, L. Wondraczek, Z. Ma, Q. Zhang and J. Qiu, J. Phys. Chem. C, 2014, 118, 7515–7522 CAS.
- Z. Xia, C. Ma, M. S. Molokeev, Q. Lin, K. Rickert and K. R. Poeppelmeier, J. Am. Chem. Soc., 2015, 137, 12494–12497 CrossRef CAS PubMed.
- Z. Xia, G. Liu, J. Wen, Z. Mei, M. Balasubramanian, M. Molokeev, L. Peng, L. Gu, D. J. Miller, Q. Liu and K. R. Poeppelmeier, J. Am. Chem. Soc., 2016, 138, 1158–1161 CrossRef CAS PubMed.
- W.-T. Chen, H.-S. Sheu, R.-S. Liu and J. P. Attfield, J. Am. Chem. Soc., 2012, 134, 8022–8025 CrossRef CAS PubMed.
- W.-Y. Huang, F. Yoshimura, K. Ueda, Y. Shimomura, H.-S. Sheu, T.-S. Chan, H. F. Greer, W. Zhou, S.-F. Hu, R.-S. Liu and J. P. Attfield, Angew. Chem., Int. Ed., 2013, 52, 8102–8106 CrossRef CAS PubMed.
- G. Li, C. C. Lin, W.-T. Chen, M. S. Molokeev, V. V. Atuchin, C.-Y. Chiang, W. Zhou, C.-W. Wang, W.-H. Li, H.-S. Sheu, T.-S. Chan, C. Ma and R.-S. Liu, Chem. Mater., 2014, 26, 2991–3001 CrossRef CAS.
- M. Peng, X. Yin, P. A. Tanner, M. G. Brik and P. Li, Chem. Mater., 2015, 27, 2938–2945 CrossRef.
- Y. Wei, X. Qi, H. Xiao, W. Luo, H. Yao, L. Lv, G. Li and J. Lin, RSC Adv., 2016, 6, 43771–43779 RSC.
- P. Arunkumar, Y. H. Kim and W. B. Im, J. Phys. Chem. C, 2016, 120, 4495–4503 CAS.
- C.-H. Huang, P.-J. Wu, J.-F. Lee and T.-M. Chen, J. Mater. Chem., 2011, 21, 10489–10495 RSC.
- G. Gao and L. Wondraczek, J. Mater. Chem. C, 2013, 1, 1952–1958 RSC.
- J. Sato, H. Kobayashi, K. Ikarashi, N. Satio, H. Nishiyama and Y. Inoue, J. Phys. Chem. B, 2004, 108, 4369–4375 CrossRef CAS.
- L. Sun, Y. Qi, C.-J. Jia, Z. Jin and W. Fan, Nanoscale, 2014, 6, 2649–2659 RSC.
- M. Shang, G. Li, D. Yang, X. Kang, C. Peng, Z. Cheng and J. Lin, Dalton Trans., 2011, 40, 9379–9387 RSC.
- S. Takeshita, J. Honda, T. Isobe, T. O. Sawayama and S. Niikura, Cryst. Growth Des., 2010, 10, 4494–4500 CAS.
- Z. Gu, F. Liu, X. Li and Z. W. Pan, Phys. Chem. Chem. Phys., 2013, 15, 7488–7493 RSC.
- M.-Y. Tsai, C.-Y. Yu, C.-C. Wang and T.-P. Perng, Cryst. Growth Des., 2008, 8, 2264–2269 CAS.
- Z. Gu, F. Liu, X. Li and Z. W. Pan, CrystEngComm, 2013, 15, 2904–2908 RSC.
- Q. Liu, Y. Zhou, Z. Tian, X. Chen, J. Gao and Z. Zou, J. Mater. Chem., 2012, 22, 2033–2038 RSC.
- S. Yan, L. Wan, Z. Li and Z. Zou, Chem. Commun., 2011, 47, 5632–5634 RSC.
- J. Liang, Y. Cao, H. Lin, Z. Zhang, C. Huang and X. Wang, Inorg. Chem., 2013, 52, 6916–6922 CrossRef CAS PubMed.
- Q. Liu, Y. Zhou, J. Kou, X. Chen, Z. Tian, J. Gao, S. Yan and Z. Zou, J. Am. Chem. Soc., 2010, 132, 14385–14387 CrossRef CAS PubMed.
- L. Zhang, X.-F. Cao, Y.-L. Ma, X.-T. Chen and Z.-L. Xue, CrystEngComm, 2010, 12, 3201–3206 RSC.
- H. He, Y. Zhang, Q. Pan, G. Wu, G. Dong and J. Qiu, J. Mater. Chem. C, 2015, 3, 5419–5429 RSC.
- Y.-J. Li and X.-P. Yan, Nanoscale, 2016, 8, 14965–14970 RSC.
- C. Larson and R. B. Von Dreele, Los Alamos National Laboratory Report LAUR, Los Alamos National Laboratory, Los Alamos, 2000, vol. 86, pp. 748–789 Search PubMed.
- Q. Bai, Z. Wang, P. Li, S. Xu, T. Li, J. Cheng and Z. Yang, Mater. Des., 2016, 108, 597–607 CrossRef CAS.
- G. Li, Y. Wang, W. Zeng, W. Chen, S. Han, H. Guo and Y. Li, J. Mater. Chem. C, 2016, 4, 3304–3312 RSC.
- P. Arunkumar, Y. H. Kim and W. B. Lm, J. Phys. Chem. C, 2016, 120, 4495–4503 CAS.
- G. Blasse and B. Grabmaier, Luminescent materials, Speringer-Verlag, 2000, vol. 3, pp. 479–493 Search PubMed.
- G. Blasse, Philips Res. Rep., 1969, 24, 131–144 CAS.
- D. L. Dexter, J. Chem. Phys., 1953, 21, 836–850 CrossRef CAS.
- G. Li, Y. Tian, Y. Zhao and J. Lin, Chem. Soc. Rev., 2015, 44, 8688–8713 RSC.
- H. S. Jang, Y.-H. Won, S. Vaidyanathan, D. H. Kim and D. Y. Jeon, J. Electrochem. Soc., 2009, 156, J138–J142 CrossRef CAS.
- Y. Shimomura, T. Honma, M. Shigeiwa, T. Akai, K. Okamoto and N. Kijima, J. Electrochem. Soc., 2007, 154, J35–J38 CrossRef CAS.
- C. K. Jorgensen, Modern aspects of ligand field theory, Amsterdam, North-Holland, 1971 Search PubMed.
- M. Shang, J. Fan, H. Lian, Y. Zhang, D. Geng and J. Lin, Inorg. Chem., 2014, 53, 7748–7755 CrossRef CAS PubMed.
- Y. Xia, J. Chen, Y. Liu, M. S. Molokeev, M. Guan, Z. Huang and M. Fang, Dalton Trans., 2016, 45, 1007–1015 RSC.
- Y. Jia, H. Qiao, Y. Zheng, N. Guo and H. You, Phys. Chem. Chem. Phys., 2012, 41, 3537–3542 RSC.
- T. Matsuzawa, Y. Aoki, N. Takeuchi and Y. Murayama, J. Electrochem. Soc., 1996, 143, 2670–2673 CrossRef CAS.
- B. Han, H. Liang, H. Ni, Q. Su, G. Yang, J. Shi and G. Zhang, Opt. Express, 2009, 17, 7138–7144 CrossRef CAS PubMed.
- J. Zhong, H. Liang, Q. Su, J. Zhou, Y. Hang and Z. G. Gao, Appl. Phys. B, 2009, 98, 139–147 CrossRef.
- P. Dorenbos, J. Lumin., 2015, 111, 89–104 CrossRef.
- L. Li, R. Li, W. Zi and S. Gan, Phys. B. Condens. Matter., 2015, 458, 8–17 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |






