Construction of highly functionalized naphthalenes using an in situ ene–allene strategy†
Abstract
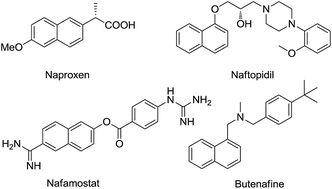
Construction of highly functionalized naphthalene derivatives remains a challenging task for organic chemists because of the effect of the substituent. In this paper, we have developed a sequence of propargyl-allenyl isomerization and electrocyclization for the synthesis of polyfunctionalized naphthalenes.

 Please wait while we load your content...
Please wait while we load your content...