Iron(iii) chloride modulated selective 1,2-trans glycosylation based on glycosyl trichloroacetimidate donors and its application in orthogonal glycosylation†
Abstract
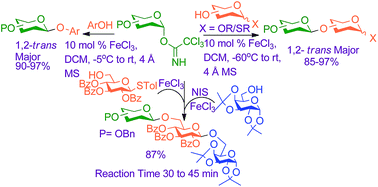
The development of a new glycosylation method for efficient stereoselective synthesis of β-gluco- and galactosides from their corresponding armed trichloroacetimidate donors mediated by 10 mole% of FeCl3 has been focused. FeCl3 has also been applied to a number of glucose, galactose, mannose and rhamnose based trichloroacetimidate donors with various protecting groups incorporated at the C-2-position to prepare a variety of disaccharides and trisaccharides with excellent 1,2-trans selectivity. FeCl3 can also modulate the 1,2-trans selectivity of the reaction of 2-O-alkylated gluco- and galacto-pyranosyl trichloroacetimidates with phenolic compounds leading to the generation of the corresponding β-O-aryl glycosides in excellent yield and selectivity. Apart from these the present methodology has been successfully utilized for double glycosylation and orthogonal glycosylation reactions along with its application in one-pot three component orthogonal glycosylation reactions for synthesis of a trisaccharide.

 Please wait while we load your content...
Please wait while we load your content...