Speciation analysis of inorganic arsenic in food and water samples by electrothermal atomic absorption spectrometry after magnetic solid phase extraction by a novel MOF-199/modified magnetite nanoparticle composite†
Abstract
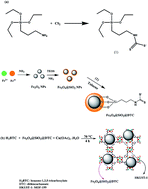
In this work a novel magnetic metal–organic framework (MOF-199/dithiocarbamate modified magnetite nanoparticle composite) was synthesized and utilized for speciation analysis of As(III) and As(V) via determination by electrothermal atomic absorption spectrometry. The synthesized sorbent represented selectivity toward As(III) at pH = 3 while As(V) remained in the initial solution. Total amount of arsenic in the samples was determined after reduction of the As(V) species to As(III) ions with the mixture of Na2S2O3 and KI. A design of experiments approach was employed to find the best extraction conditions by evaluating the parameters affecting the preconcentration procedure. Under the optimal conditions the limit of detection, linearity and relative standard deviation of the method for As(III) were 1.2 ng L−1, 4–300 ng L−1 and <8.4%, respectively. The developed method was validated by analyzing two certified reference materials. Finally, the outlined method was successfully employed to the rapid extraction and speciation analysis of As(III) and As(V) in water samples and total arsenic in rice and canned tuna samples.

 Please wait while we load your content...
Please wait while we load your content...